Long-term synaptic plasticity is impaired in rats with lesions of the ventrolateral preoptic nucleus
- PMID: 20128848
- PMCID: PMC2819366
- DOI: 10.1111/j.1460-9568.2009.07001.x
Long-term synaptic plasticity is impaired in rats with lesions of the ventrolateral preoptic nucleus
Abstract
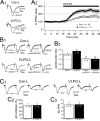
Impairment of memory functions has been frequently reported in models of sleep deprivation. Similarly, hippocampal long-term synaptic plasticity has been shown to be sensitive to sleep loss caused by acute sleep restriction. However, such approaches are limited by the stressful nature of sleep deprivation, and because it is difficult to study long-term sleep restriction in animals. Here, we report the effects of chronic sleep loss on hippocampal long-term potentiation (LTP) in a rodent model of chronic partial sleep deprivation. We studied LTP of the Schaffer collateral-CA1 synapses in hippocampal slices prepared from rats with lesions of the ventrolateral preoptic nucleus (VLPO), which suffered reductions in total sleep time for several weeks after lesions. In slices prepared from VLPO-lesioned rats, LTP was impaired proportionally to the amount of sleep loss, and the decline in LTP followed a single exponential function over the amount of accumulated sleep debt. As compared with sham-lesioned controls, hippocampal slices from VLPO-lesioned rats showed a greater response to adenosine antagonists and greater paired-pulse facilitation (PPF). However, exogenous adenosine depressed evoked synaptic transmission and increased PPF in VLPO-lesioned and sham-lesioned rats by equal amounts, suggesting that the greater endogenous adenosine inhibitory tone in the VLPO-lesioned rats is associated with greater ligand accumulation rather than a change in adenosine receptor sensitivity or adenosine-mediated neurotransmitter release probability. LTP in VLPO-lesioned animals was partially restored by adenosine antagonists, suggesting that adenosine accumulation in VLPO-lesioned animals could account for some of the observed synaptic plasticity deficits.
Figures
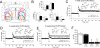



References
-
- Alanko L, Heiskanen S, Stenberg D, Porkka-Heiskanen T. Adenosine kinase and 5'-nucleotidase activity after prolonged wakefulness in the cortex and the basal forebrain of rat. Neurochem Int. 2003a;42:449–454. - PubMed
-
- Alanko L, Stenberg D, Porkka-Heiskanen T. Nitrobenzylthioinosine (NBMPR) binding and nucleoside transporter ENT1 mRNA expression after prolonged wakefulness and recovery sleep in the cortex and basal forebrain of rat. J Sleep Res. 2003b;12:299–304. - PubMed
-
- Arai A, Kessler M, Lynch G. The effects of adenosine on the development of long-term potentiation. Neurosci Lett. 1990;119:41–44. - PubMed
-
- Arai A, Lynch G. Factors regulating the magnitude of long-term potentiation induced by theta pattern stimulation. Brain Res. 1992;598:173–184. - PubMed
-
- Arrigoni E, Rosenberg PA. Nitric oxide-induced adenosine inhibition of hippocampal synaptic transmission depends on adenosine kinase inhibition and is cyclic GMP independent. Eur J Neurosci. 2006;24:2471–2480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

