Heterologous expression of leader-less pga gene in Pichia pastoris: intracellular production of prokaryotic enzyme
- PMID: 20128906
- PMCID: PMC2845550
- DOI: 10.1186/1472-6750-10-7
Heterologous expression of leader-less pga gene in Pichia pastoris: intracellular production of prokaryotic enzyme
Abstract
Background: Penicillin G acylase of Escherichia coli (PGAEc) is a commercially valuable enzyme for which efficient bacterial expression systems have been developed. The enzyme is used as a catalyst for the hydrolytic production of beta-lactam nuclei or for the synthesis of semi-synthetic penicillins such as ampicillin, amoxicillin and cephalexin. To become a mature, periplasmic enzyme, the inactive prepropeptide of PGA has to undergo complex processing that begins in the cytoplasm (autocatalytic cleavage), continues at crossing the cytoplasmic membrane (signal sequence removing), and it is completed in the periplasm. Since there are reports on impressive cytosolic expression of bacterial proteins in Pichia, we have cloned the leader-less gene encoding PGAEc in this host and studied yeast production capacity and enzyme authenticity.
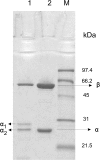
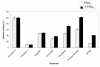
Results: Leader-less pga gene encoding PGAEcunder the control of AOX1 promoter was cloned in Pichia pastoris X-33. The intracellular overproduction of heterologous PGAEc(hPGAEc) was evaluated in a stirred 10 litre bioreactor in high-cell density, fed batch cultures using different profiles of transient phases. Under optimal conditions, the average volumetric activity of 25900 U l-1 was reached. The hPGAEc was purified, characterized and compared with the wild-type PGAEc. The alpha-subunit of the hPGAEc formed in the cytosol was processed aberrantly resulting in two forms with C- terminuses extended to the spacer peptide. The enzyme exhibited modified traits: the activity of the purified enzyme was reduced to 49%, the ratios of hydrolytic activities with cephalexin, phenylacetamide or 6-nitro-3-phenylacetylamidobenzoic acid (NIPAB) to penicillin G increased and the enzyme showed a better synthesis/hydrolysis ratio for the synthesis of cephalexin.
Conclusions: Presented results provide useful data regarding fermentation strategy, intracellular biosynthetic potential, and consequences of the heterologous expression of PGAEc in P. pastoris X-33. Aberrant processing of the precursor of PGAEc in the cytosol yielded the mature enzyme with modified traits.
Figures

Similar articles
-
Potential of Pichia pastoris for the production of industrial penicillin G acylase.Folia Microbiol (Praha). 2017 Sep;62(5):417-424. doi: 10.1007/s12223-017-0512-0. Epub 2017 Mar 9. Folia Microbiol (Praha). 2017. PMID: 28281229
-
[Constitutive expression and purification of Alcaligenes faecalis penicillin G acylase in Escherichia coli].Sheng Wu Gong Cheng Xue Bao. 2004 Sep;20(5):736-40. Sheng Wu Gong Cheng Xue Bao. 2004. PMID: 15974000 Chinese.
-
High-level production and covalent immobilization of Providencia rettgeri penicillin G acylase (PAC) from recombinant Pichia pastoris for the development of a novel and stable biocatalyst of industrial applicability.Biotechnol Bioeng. 2006 Feb 5;93(2):344-54. doi: 10.1002/bit.20728. Biotechnol Bioeng. 2006. PMID: 16259000
-
Biotechnological advances on penicillin G acylase: pharmaceutical implications, unique expression mechanism and production strategies.Biotechnol Adv. 2013 Dec;31(8):1319-32. doi: 10.1016/j.biotechadv.2013.05.006. Epub 2013 May 27. Biotechnol Adv. 2013. PMID: 23721991 Review.
-
Process technology for production and recovery of heterologous proteins with Pichia pastoris.Biotechnol Prog. 2006 Nov-Dec;22(6):1465-73. doi: 10.1021/bp060171t. Biotechnol Prog. 2006. PMID: 17137292 Review.
Cited by
-
Production and secretion dynamics of prokaryotic Penicillin G acylase in Pichia pastoris.Appl Microbiol Biotechnol. 2020 Jul;104(13):5787-5800. doi: 10.1007/s00253-020-10669-x. Epub 2020 May 18. Appl Microbiol Biotechnol. 2020. PMID: 32424437 Free PMC article.
-
Heterologous expression of recombinant urate oxidase using the intein-mediated protein purification in Pichia pastoris.3 Biotech. 2021 Mar;11(3):120. doi: 10.1007/s13205-021-02670-6. Epub 2021 Feb 8. 3 Biotech. 2021. PMID: 33628707 Free PMC article.
-
Potential of Pichia pastoris for the production of industrial penicillin G acylase.Folia Microbiol (Praha). 2017 Sep;62(5):417-424. doi: 10.1007/s12223-017-0512-0. Epub 2017 Mar 9. Folia Microbiol (Praha). 2017. PMID: 28281229
-
Efficient extracellular expression and rapid screening of human-like recombinant gelatin in Komagataella phaffii.Sci Rep. 2025 Aug 18;15(1):30251. doi: 10.1038/s41598-025-14855-7. Sci Rep. 2025. PMID: 40826158 Free PMC article.
-
A Proposal for Six Sigma Integration for Large-Scale Production of Penicillin G and Subsequent Conversion to 6-APA.J Anal Methods Chem. 2014;2014:413616. doi: 10.1155/2014/413616. Epub 2014 Feb 24. J Anal Methods Chem. 2014. PMID: 25057428 Free PMC article. Review.
References
-
- Rajendhran J, Gunasekaran P. Recent biotechnological interventions for developing improved penicillin G acylases. J Biosci Bioeng. 2004;97:1–13. - PubMed
-
- Sobotková L, Plháčková K, Kyslík P, Vojtíšek V. Czech Patent No. 278516. 1993.
-
- Kutzbach C, Rauenbusch E. Preparation and general properties of crystalline penicillin acylase from Escherichia coli ATCC 11105. Hoppe-Seylers Z Physiol Chem. 1974;354:45–53. - PubMed
-
- Robak M, Szewczuk A. Penicillin amidase from Proteus rettgeri. Acta Biochim Pol. 1981;28:275–284. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

