Level of response and safety of pharmacological monotherapy in the treatment of acute bipolar I disorder phases: a systematic review and meta-analysis
- PMID: 20128953
- PMCID: PMC3005373
- DOI: 10.1017/S1461145709991246
Level of response and safety of pharmacological monotherapy in the treatment of acute bipolar I disorder phases: a systematic review and meta-analysis
Abstract
In recent years, combinations of pharmacological treatments have become common for the treatment of bipolar disorder type I (BP I); however, this practice is usually not evidence-based and rarely considers monotherapy drug regimen (MDR) as an option in the treatment of acute phases of BP I. Therefore, we evaluated comparative data of commonly prescribed MDRs for both manic and depressive phases of BP I. Medline, PsycINFO, EMBASE, the Cochrane Library, the ClinicalStudyResults.org and other data sources were searched from 1949 to March 2009 for placebo and active controlled randomized clinical trials (RCTs). Risk ratios (RRs) for response, remission, and discontinuation rates due to adverse events (AEs), lack of efficacy, or discontinuation due to any cause, and the number needed to treat or harm (NNT or NNH) were calculated for each medication individually and for all evaluable trials combined. The authors included 31 RCTs in the analyses comparing a MDR with placebo or with active treatment for acute mania, and 9 RCTs comparing a MDR with placebo or with active treatment for bipolar depression. According to the collected evidence, most of the MDRs when compared to placebo showed significant response and remission rates in acute mania. In the case of bipolar depression only quetiapine and, to a lesser extent, olanzapine showed efficacy as MDR. Overall, MDRs were well tolerated with low discontinuation rates due to any cause or AE, although AE profiles differed among treatments. We concluded that most MDRs were efficacious and safe in the treatment of manic episodes, but very few MDRs have demonstrated being efficacious for bipolar depressive episodes.
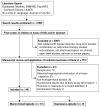
Figures


Similar articles
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Pharmacological treatments in panic disorder in adults: a network meta-analysis.Cochrane Database Syst Rev. 2023 Nov 28;11(11):CD012729. doi: 10.1002/14651858.CD012729.pub3. Cochrane Database Syst Rev. 2023. PMID: 38014714 Free PMC article.
-
Electronic cigarettes for smoking cessation.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD010216. doi: 10.1002/14651858.CD010216.pub7. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 Jan 8;1:CD010216. doi: 10.1002/14651858.CD010216.pub8. PMID: 36384212 Free PMC article. Updated.
Cited by
-
Role of quetiapine beyond its clinical efficacy in bipolar disorder: From neuroprotection to the treatment of psychiatric disorders (Review).Exp Ther Med. 2015 Mar;9(3):643-652. doi: 10.3892/etm.2015.2213. Epub 2015 Jan 23. Exp Ther Med. 2015. PMID: 25667608 Free PMC article.
-
Acetazolamide for Bipolar Disorders: A Scoping Review.Brain Sci. 2023 Jan 13;13(1):140. doi: 10.3390/brainsci13010140. Brain Sci. 2023. PMID: 36672121 Free PMC article.
-
Drug treatment patterns in bipolar disorder: analysis of long-term self-reported data.Int J Bipolar Disord. 2013 May 3;1:5. doi: 10.1186/2194-7511-1-5. eCollection 2013. Int J Bipolar Disord. 2013. PMID: 25505672 Free PMC article.
-
Brain stimulation treatment for bipolar disorder.Bipolar Disord. 2023 Feb;25(1):9-24. doi: 10.1111/bdi.13283. Epub 2022 Dec 21. Bipolar Disord. 2023. PMID: 36515461 Free PMC article. Review.
-
Efficacy of pharmacotherapy in bipolar disorder: a report by the WPA section on pharmacopsychiatry.Eur Arch Psychiatry Clin Neurosci. 2012 Jun;262 Suppl 1:1-48. doi: 10.1007/s00406-012-0323-x. Eur Arch Psychiatry Clin Neurosci. 2012. PMID: 22622948
References
-
- Amsterdam JD, Shults J. Comparison of fluoxetine, olanzapine, and combined fluoxetine plus olanzapine initial therapy of bipolar type I and type II major depression–lack of manic induction. Journal of Affective Disorders. 2005;87:121–130. - PubMed
-
- Baldessarini RJ, Leahy L, Arcona S, Gause D, et al. Patterns of psychotropic drug prescription for U.S. patients with diagnoses of bipolar disorders. Psychiatry Services. 2007;58:85–91. - PubMed
-
- Ballenger JC, Post RM. Therapeutic effects of carbamazepine in affective illness: a preliminary report. Communications in Psychopharmacology. 1978;2:159–175. - PubMed
-
- Ballenger JC, Post RM. Carbamazepine in manic-depressive illness: a new treatment. American Journal of Psychiatry. 1980;137:782–790. - PubMed
-
- Baron M, Gershon ES, Rudy V, Jonas WZ, et al. Lithium carbonate response in depression. Prediction by unipolar/bipolar illness, average-evoked response, catechol-O-methyl transferase, and family history. Archives of General Psychiatry. 1975;32:1107–1111. - PubMed

