Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation
- PMID: 20129250
- PMCID: PMC2818776
- DOI: 10.1016/j.ccr.2009.12.008
Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation
Abstract
Chronic exposure to tobacco smoke, which contains over 60 tumor-initiating carcinogens, is the major risk factor for development of lung cancer, accounting for a large portion of cancer-related deaths worldwide. It is well established that tobacco smoke is a tumor initiator, but we asked whether it also acts as a tumor promoter once malignant initiation, such as caused by K-ras activation, has taken place. Here we demonstrate that repetitive exposure to tobacco smoke promotes tumor development both in carcinogen-treated mice and in transgenic mice undergoing sporadic K-ras activation in lung epithelial cells. Tumor promotion is due to induction of inflammation that results in enhanced pneumocyte proliferation and is abrogated by IKKbeta ablation in myeloid cells or inactivation of JNK1.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures
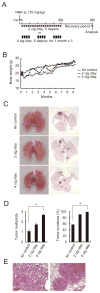
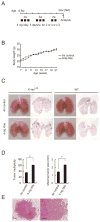
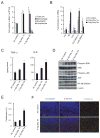

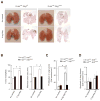

References
-
- Berns A. Cancer. Improved mouse models. Nature. 2001;410:1043–1044. - PubMed
-
- Borm PJ, Driscoll K. Particles, inflammation and respiratory tract carcinogenesis. Toxicol Lett. 1996;88:109–113. - PubMed
-
- Brown BG, Chang CJ, Ayres PH, Lee CK, Doolittle DJ. The effect of cotinine or cigarette smoke co-administration on the formation of O6-methylguanine adducts in the lung and liver of A/J mice treated with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) Toxicol Sci. 1999;47:33–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

