An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing
- PMID: 20129283
- PMCID: PMC2822446
- DOI: 10.1016/j.hrthm.2009.09.069
An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing
Abstract
Background: Brugada syndrome (BrS) is a common heritable channelopathy. Mutations in the SCN5A-encoded sodium channel (BrS1) culminate in the most common genotype.
Objective: This study sought to perform a retrospective analysis of BrS databases from 9 centers that have each genotyped >100 unrelated cases of suspected BrS.
Methods: Mutational analysis of all 27 translated exons in SCN5A was performed. Mutation frequency, type, and localization were compared among cases and 1,300 ostensibly healthy volunteers including 649 white subjects and 651 nonwhite subjects (blacks, Asians, Hispanics, and others) that were genotyped previously.
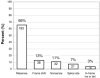
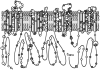
Results: A total of 2,111 unrelated patients (78% male, mean age 39 +/- 15 years) were referred for BrS genetic testing. Rare mutations/variants were more common among BrS cases than control subjects (438/2,111, 21% vs. 11/649, 1.7% white subjects and 31/651, 4.8% nonwhite subjects, respectively, P <10(-53)). The yield of BrS1 genetic testing ranged from 11% to 28% (P = .0017). Overall, 293 distinct mutations were identified in SCN5A: 193 missense, 32 nonsense, 38 frameshift, 21 splice-site, and 9 in-frame deletions/insertions. The 4 most frequent BrS1-associated mutations were E1784K (14x), F861WfsX90 (11x), D356N (8x), and G1408R (7x). Most mutations localized to the transmembrane-spanning regions.
Conclusion: This international consortium of BrS genetic testing centers has added 200 new BrS1-associated mutations to the public domain. Overall, 21% of BrS probands have mutations in SCN5A compared to the 2% to 5% background rate of rare variants reported in healthy control subjects. Additional studies drawing on the data presented here may help further distinguish pathogenic mutations from similarly rare but otherwise innocuous ones found in cases.
Figures



Comment in
-
Brugada syndrome: lots of questions, some answers.Heart Rhythm. 2010 Jan;7(1):47-9. doi: 10.1016/j.hrthm.2009.10.016. Heart Rhythm. 2010. PMID: 20129284 No abstract available.
-
The SCN5A gene in Brugada syndrome: mutations, variants, missense and nonsense. What's a clinician to do?Heart Rhythm. 2010 Jan;7(1):50-1. doi: 10.1016/j.hrthm.2009.10.019. Epub 2009 Oct 17. Heart Rhythm. 2010. PMID: 20129285 No abstract available.
-
To the editor--the compendium of SCN5A mutations.Heart Rhythm. 2010 Apr;7(4):e1. doi: 10.1016/j.hrthm.2010.01.037. Epub 2010 Feb 1. Heart Rhythm. 2010. PMID: 20188230 No abstract available.
References
-
- Chen PS, Priori SG. The Brugada syndrome. J Am Coll Cardiol. 2008;51:1176–1180. - PubMed
-
- Meregalli PG, Wilde AAM, Tan HL. Pathophysiological mechanisms of Brugada syndrome: depolarization disorder, repolarization disorder, or more? Cardiovasc Res. 2005;67:367–378. - PubMed
-
- Antzelevitch C, Brugada P, Borggrefe M, et al. Brugada syndrome: report of the second consensus conference. Heart Rhythm. 2005;2:429–440. Erratum: Heart Rhythm 2005;2:905. - PubMed
-
- Tester DJ, Ackerman MJ. Cardiomyopathic and channelopathic causes of sudden unexplained death in infants and children. Annu Rev Med. 2009;60:69–84. - PubMed
-
- Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

