A 3-D cardiac muscle construct for exploring adult marrow stem cell based myocardial regeneration
- PMID: 20129663
- PMCID: PMC2887929
- DOI: 10.1016/j.biomaterials.2010.01.041
A 3-D cardiac muscle construct for exploring adult marrow stem cell based myocardial regeneration
Abstract
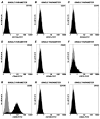
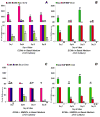
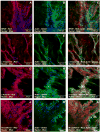
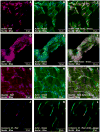
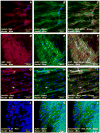
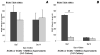
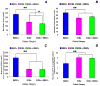
Adult bone marrow stromal cells (BMSCs) are capable of differentiating into cardiomyocyte-like cells in vitro and contribute to myocardial regeneration in vivo. Consequently, BMSCs may potentially play a vital role in cardiac repair and regeneration. However, this concept has been limited by inadequate and inconsistent differentiation of BMSCs into cardiomyocytes along with poor survival and integration of neo-cardiomyocytes after implantation into ischemic myocardium. In order to overcome these barriers and to explore adult stem cell based myocardial regeneration, we have developed an in vitro model of three-dimensional (3-D) cardiac muscle using rat ventricular embryonic cardiomyocytes (ECMs) and BMSCs. When ECMs and BMSCs were seeded sequentially onto a 3-D tubular scaffold engineered from topographically aligned type I collagen-fibers and cultured in basal medium for 7, 14, 21, or 28 days, the maturation and co-differentiation into a cardiomyocyte lineage was observed. Phenotypic induction was characterized at morphological, immunological, biochemical and molecular levels. The observed expression of transcripts coding for cardiomyocyte phenotypic markers and the immunolocalization of cardiomyogenic lineage-associated proteins revealed typical expression patterns of neo-cardiomyogenesis. At the biochemical level differentiating cells exhibited appropriate metabolic activity and at the ultrastructural level myofibrillar and sarcomeric organization were indicative of an immature phenotype. Our 3-D co-culture system sustains the ECMs in vitro continuum of differentiation process and simultaneously induces the maturation and differentiation of BMSCs into cardiomyocyte-like cells. Thus, this novel 3-D co-culture system provides a useful in vitro model to investigate the functional role and interplay of developing ECMs and BMSCs during cardiomyogenic differentiation.
Copyright 2010 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures



References
-
- Korbling M, Estrov Z. Adult stem cells for tissue repair: A new therapeutic concept? N Engl J Med. 2003;349:570–582. - PubMed
-
- Bunting KD, Hawley RG. Integrative molecular and developmental biology of adult stem cells. Biol Cell. 2003;95:563–578. - PubMed
-
- Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18:1417–26. - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

