Investigation of mechanism(s) of DNA damage induced by 4-monochlorobiphenyl (PCB3) metabolites
- PMID: 20129669
- PMCID: PMC2888624
- DOI: 10.1016/j.envint.2009.12.004
Investigation of mechanism(s) of DNA damage induced by 4-monochlorobiphenyl (PCB3) metabolites
Abstract
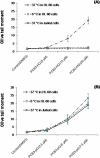
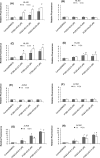
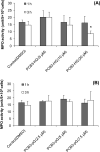
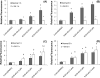
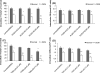
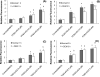
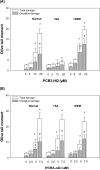
4-Monochlorobiphenyl (PCB3) is readily converted by xenobiotic-metabolizing enzymes to dihydroxy-metabolites and quinones. The PCB3 hydroquinone (PCB3-HQ; 2-(4'-chlorophenyl)-1,4-hydroquinone) induces chromosome loss in Chinese Hamster V79 cells, whereas the para-quinone (PCB3-pQ; 2-(4'-chlorophenyl)-1,4-benzoquinone) very efficiently induces gene mutations and chromosome breaks. Apparently, each of these two metabolites, which are a redox pair, has a different spectrum of genotoxic effects due to different, metabolite-specific mechanisms. We hypothesized that the HQ requires enzymatic activation by peroxidases with the formation of reactive oxygen species (ROS) as the ultimate genotoxin, whereas the pQ reacts directly with nucleophilic sites in DNA and/or proteins. To examine this hypothesis, we employed two cell lines with different myeloperoxidase (MPO) activities, MPO-rich HL-60 and MPO-deficient Jurkat cells, and measured cytotoxicity, DNA damage (COMET assay), MPO activity, intracellular levels of reactive oxygen species (ROS) and intracellular free -SH groups (monochlorobimane assay, MCB) and free GSH contents (enzyme recycling method) after treatment with PCB3-HQ and PCB3-pQ. We also examined the modulation of these effects by normal/low temperature, pre-treatment with an MPO inhibitor (succinylacetone, SA), or GSH depletion. PCB3-p-Q increased intracellular ROS levels and induced DNA damage in both HL-60 and Jurkat cells at 37°C and 6°C, indicating a direct, MPO-independent mode of activity. It also strongly reduced intracellular free -SH groups and GSH levels in normal and GSH-depleted cells. Thus the ROS increase could be caused by reduced protection by GSH or non-enzymatic autoxidation of the resulting PCB3-HQ-GSH adduct. PCB3-HQ did not produce a significant reduction of intracellular GSH in HL-60 cells and reduced intracellular free -SH groups only at the highest concentration tested in GSH depleted cells. Moreover, PCB3-HQ induced DNA damage and ROS production only at 37 °C in HL-60 cells, not at 6 °C or in Jurkat cells at either temperature; no significant DNA damage and ROS production was observed in HL-60 cells at 37 °C if MPO activity was inhibited by SA. These studies show that the effects of PCB3-HQ are enzyme dependent, i.e. PCB3-HQ is oxidized by MPO in HL-60 cells with the generation of ROS and induction of DNA damage. However, this is not the case with the PCB3-pQ, which may produce DNA damage by the reactivity of the quinone with the DNA or nuclear proteins, or possibly by indirectly increasing intracellular ROS levels by GSH depletion. These different modes of action explain not only the different types of genotoxicity observed previously, but also suggest different organ specificity of these genotoxins.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Quinoid metabolites of 4-monochlorobiphenyl induce gene mutations in cultured Chinese hamster v79 cells.Toxicol Sci. 2007 Nov;100(1):88-98. doi: 10.1093/toxsci/kfm204. Epub 2007 Aug 8. Toxicol Sci. 2007. PMID: 17686921
-
Polyploidy-induction by dihydroxylated monochlorobiphenyls: structure-activity-relationships.Environ Int. 2010 Nov;36(8):962-9. doi: 10.1016/j.envint.2010.03.012. Epub 2010 May 14. Environ Int. 2010. PMID: 20471090 Free PMC article.
-
Hydroquinone-induced genotoxicity and oxidative DNA damage in HepG2 cells.Chem Biol Interact. 2008 May 9;173(1):1-8. doi: 10.1016/j.cbi.2008.02.002. Epub 2008 Feb 14. Chem Biol Interact. 2008. PMID: 18358459
-
Induction of cytochromes P450, caspase-3 and DNA damage by PCB3 and its hydroxylated metabolites in porcine ovary.Toxicol Lett. 2006 Oct 25;166(3):200-11. doi: 10.1016/j.toxlet.2006.07.304. Epub 2006 Jul 14. Toxicol Lett. 2006. PMID: 16949219
-
Use of human derived liver cells for the detection of genotoxins in comet assays.Mutat Res Genet Toxicol Environ Mutagen. 2019 Sep;845:402995. doi: 10.1016/j.mrgentox.2018.12.003. Epub 2018 Dec 10. Mutat Res Genet Toxicol Environ Mutagen. 2019. PMID: 31561885 Review.
Cited by
-
Polychlorinated biphenyls (PCBs) as initiating agents in hepatocellular carcinoma.Cancer Lett. 2013 Jun 28;334(1):46-55. doi: 10.1016/j.canlet.2012.11.041. Epub 2012 Dec 2. Cancer Lett. 2013. PMID: 23211541 Free PMC article. Review.
-
3,3'-Dichlorobiphenyl Is Metabolized to a Complex Mixture of Oxidative Metabolites, Including Novel Methoxylated Metabolites, by HepG2 Cells.Environ Sci Technol. 2020 Oct 6;54(19):12345-12357. doi: 10.1021/acs.est.0c03476. Epub 2020 Sep 23. Environ Sci Technol. 2020. PMID: 32910851 Free PMC article.
-
Correlating blood levels of 8-hydroxydeoxyguanosine to hOGG1 genotypes and the incidence of ischemic cardiomyopathy.Kaohsiung J Med Sci. 2016 May;32(5):241-7. doi: 10.1016/j.kjms.2016.04.010. Epub 2016 Jun 2. Kaohsiung J Med Sci. 2016. PMID: 27316582 Free PMC article.
-
Metabolism and metabolites of polychlorinated biphenyls.Crit Rev Toxicol. 2015 Mar;45(3):245-72. doi: 10.3109/10408444.2014.999365. Epub 2015 Jan 28. Crit Rev Toxicol. 2015. PMID: 25629923 Free PMC article. Review.
-
Atmospheric dispersion of PCB from a contaminated Lake Michigan harbor.Atmos Environ (1994). 2015 Dec 1;122:791-798. doi: 10.1016/j.atmosenv.2015.10.040. Atmos Environ (1994). 2015. PMID: 26594127 Free PMC article.
References
-
- Amaro AR, Oakley GG, Bauer U, Spielmann HP, Robertson LW. Metabolic Activation of PCBs to Quinones: Reactivity toward Nitrogen and Sulfur Nucleophiles and Influence of Superoxide Dismutase. Chem. Res. Toxicol. 1996;9(3):623–629. - PubMed
-
- Arlt VM, Henderson CJ, Wolf CR, Schmeiser HH, Phillips DH, Stiborova M. Bioactivation of 3-aminobenzanthrone, a human metabolite of the environmental pollutant 3-nitrobenzanthrone: evidence for DNA adduct formation mediated by cytochrome P450 enzymes and peroxidases. Cancer Lett. 2006;234(2):220–31. - PubMed
-
- ATSDR Toxicological Profile for Polychlorinated Biphenyls. 2000. - PubMed
-
- Babior BM. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978;298(13):721–5. - PubMed
-
- Baker MA, Cerniglia GJ, Zaman A. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Analytical Biochemistry. 1990;190(2):360–365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

