BMP-2 and TGF-beta1 differentially control expression of type II procollagen and alpha 10 and alpha 11 integrins in mouse chondrocytes
- PMID: 20129696
- PMCID: PMC3791590
- DOI: 10.1016/j.ejcb.2009.10.018
BMP-2 and TGF-beta1 differentially control expression of type II procollagen and alpha 10 and alpha 11 integrins in mouse chondrocytes
Abstract
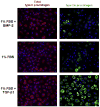
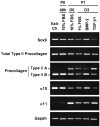
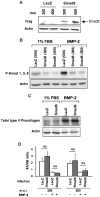
Bone morphogenetic protein (BMP)-2 and transforming growth factor (TGF)-beta1 are multifunctional cytokines both proposed as stimulants for cartilage repair. Thus it is crucial to closely examine and compare their effects on the expression of key markers of the chondrocyte phenotype, at the gene and protein level. In this study, the expression of alpha 10 and alpha 11 integrin subunits and the IIA/IIB spliced forms of type II procollagen have been monitored for the first time in parallel in the same in vitro model of mouse chondrocyte dedifferentiation/redifferentiation. We demonstrated that TGF-beta1 stimulates the expression of the non-chondrogenic form of type II procollagen, IIA isoform, and of a marker of mesenchymal tissues, i.e. the alpha 11 integrin subunit. On the contrary, BMP-2 stimulates the cartilage-specific form of type II procollagen, IIB isoform, and a specific marker of chondrocytes, i.e. the alpha 10 integrin subunit. Collectively, our results demonstrate that BMP-2 has a better capability than TGF-beta1 to stimulate chondrocyte redifferentiation and reveal that the relative expressions of type IIB to type IIA procollagens and alpha 10 to alpha 11 integrin subunits are good markers to define the differentiation state of chondrocytes. In addition, adenoviral expression of Smad6, an inhibitor of BMP canonical Smad signaling, did not affect expression of total type II procollagen or the ratio of type IIA and type IIB isoforms in mouse chondrocytes exposed to BMP-2. This result strongly suggests that signaling pathways other than Smad proteins are involved in the effect of BMP-2 on type II procollagen expression.
(c) 2010 Elsevier GmbH. All rights reserved.
Figures



Similar articles
-
[Human chondrocyte responsiveness to bone morphogenetic protein-2 after their in vitro dedifferentiation: potential use of bone morphogenetic protein-2 for cartilage cell therapy].Pathol Biol (Paris). 2009 Jun;57(4):282-9. doi: 10.1016/j.patbio.2008.04.013. Epub 2008 Jun 6. Pathol Biol (Paris). 2009. PMID: 18538953 French.
-
Alternative splicing of type II procollagen pre-mRNA in chondrocytes is oppositely regulated by BMP-2 and TGF-beta1.FEBS Lett. 2003 Jun 19;545(2-3):115-9. doi: 10.1016/s0014-5793(03)00510-6. FEBS Lett. 2003. PMID: 12804760
-
A unique tool to selectively detect the chondrogenic IIB form of human type II procollagen protein.Matrix Biol. 2014 Feb;34:80-8. doi: 10.1016/j.matbio.2013.09.001. Epub 2013 Sep 17. Matrix Biol. 2014. PMID: 24055103
-
Modulation of collagen synthesis in normal and osteoarthritic cartilage.Biorheology. 2004;41(3-4):535-42. Biorheology. 2004. PMID: 15299284 Review.
-
Alternative splicing of type II procollagen: IIB or not IIB?Connect Tissue Res. 2014 Jun;55(3):165-76. doi: 10.3109/03008207.2014.908860. Epub 2014 Apr 18. Connect Tissue Res. 2014. PMID: 24669942 Free PMC article. Review.
Cited by
-
Dynamic compression of chondrocyte-agarose constructs reveals new candidate mechanosensitive genes.PLoS One. 2012;7(5):e36964. doi: 10.1371/journal.pone.0036964. Epub 2012 May 17. PLoS One. 2012. PMID: 22615857 Free PMC article.
-
Cytokine networking of chondrocyte dedifferentiation in vitro and its implications for cell-based cartilage therapy.Am J Transl Res. 2015 Feb 15;7(2):194-208. eCollection 2015. Am J Transl Res. 2015. PMID: 25901191 Free PMC article. Review.
-
A review of advanced hydrogels for cartilage tissue engineering.Front Bioeng Biotechnol. 2024 Feb 8;12:1340893. doi: 10.3389/fbioe.2024.1340893. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38390359 Free PMC article. Review.
-
BMP-2, hypoxia, and COL1A1/HtrA1 siRNAs favor neo-cartilage hyaline matrix formation in chondrocytes.Tissue Eng Part C Methods. 2015 Feb;21(2):133-47. doi: 10.1089/ten.TEC.2013.0724. Epub 2014 Jul 31. Tissue Eng Part C Methods. 2015. PMID: 24957638 Free PMC article.
-
Zone-Dependent Architecture and Biochemical Composition of Decellularized Porcine Nasal Cartilage Modulate the Activity of Adipose Tissue-Derived Stem Cells in Cartilage Regeneration.Int J Mol Sci. 2021 Sep 14;22(18):9917. doi: 10.3390/ijms22189917. Int J Mol Sci. 2021. PMID: 34576079 Free PMC article.
References
-
- Aigner T, Zhu Y, Chansky HH, Matsen FA, 3rd, Maloney WJ, Sandell LJ. Reexpression of type IIA procollagen by adult articular chondrocytes in osteoarthritic cartilage. Arthritis Rheum. 1999;42:1443–1450. - PubMed
-
- Blaney Davidson EN, van der Kraan PM, van den Berg WB. TGF-beta and osteoarthritis. Osteoarthritis Cartilage. 2007;15:597–604. - PubMed
-
- Camper L, Hellman U, Lundgren-Akerlund E. Isolation, cloning, and sequence analysis of the integrin subunit alpha10, a beta1-associated collagen binding integrin expressed on chondrocytes. J Biol Chem. 1998;273:20383–20389. - PubMed
-
- Camper L, Holmvall K, Wangnerud C, Aszodi A, Lundgren-Akerlund E. Distribution of the collagen-binding integrin alpha10beta1 during mouse development. Cell Tissue Res. 2001;306:107–116. - PubMed
-
- Cheah KS, Lau ET, Au PK, Tam PP. Expression of the mouse alpha 1(II) collagen gene is not restricted to cartilage during development. Development. 1991;111:945–953. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

