Ligand-induced rearrangements of the GABA(B) receptor revealed by fluorescence resonance energy transfer
- PMID: 20129919
- PMCID: PMC2856234
- DOI: 10.1074/jbc.M109.077990
Ligand-induced rearrangements of the GABA(B) receptor revealed by fluorescence resonance energy transfer
Abstract
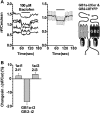
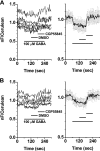
The gamma-aminobutyric acid type B receptor (GABA(B)R), one of the family C G-protein-coupled receptor members, exists as a heterodimer comprised of subunits GB1 and GB2. To clarify the ligand-induced activation mechanism of the GABA(B)R, each subunit was fused with either Cerulean or enhanced yellow fluorescent protein at its intracellular loop, and fluorescence resonance energy transfer (FRET) changes upon agonist application were monitored. As a result, FRET decreases were observed between GB1a loop 2 and GB2 loop 2 and between GB1a loop 2 and GB2 loop 1, suggesting the dissociation of intracellular domains during the receptor activation. Both intersubunit FRET pairs were expected to faithfully capture the activation of the original receptor as their pharmacological properties were highly similar to that of the wild-type receptor. However, the intrasubunit data suggest that the receptor activation does not involve major structural changes within the transmembrane domain of each subunit. By combining the results obtained from two different levels, it was concluded that the GABA(B)R activation by agonist is associated with an asymmetrical intersubunit rearrangement of GB1a and GB2 on the membrane. This type of activation mode, an intersubunit rearrangement without apparent intrahelical structural changes, appears commonly shared by the GABA(B)R and the metabotropic glutamate receptor 1alpha, another family C G-protein-coupled receptor previously studied by our group. Nevertheless, the directions of intracellular domain movements and its asymmetry observed here highlight the qualitative difference between the two receptors.
Figures






Similar articles
-
Coexpression of full-length gamma-aminobutyric acid(B) (GABA(B)) receptors with truncated receptors and metabotropic glutamate receptor 4 supports the GABA(B) heterodimer as the functional receptor.J Pharmacol Exp Ther. 2000 May;293(2):460-7. J Pharmacol Exp Ther. 2000. PMID: 10773016
-
The intracellular loops of the GB2 subunit are crucial for G-protein coupling of the heteromeric gamma-aminobutyrate B receptor.Mol Pharmacol. 2002 Aug;62(2):343-50. doi: 10.1124/mol.62.2.343. Mol Pharmacol. 2002. PMID: 12130687
-
A single subunit (GB2) is required for G-protein activation by the heterodimeric GABA(B) receptor.J Biol Chem. 2002 Feb 1;277(5):3236-41. doi: 10.1074/jbc.M108900200. Epub 2001 Nov 15. J Biol Chem. 2002. PMID: 11711539 Free PMC article.
-
Gatekeepers Controlling GPCR Export and Function.Trends Pharmacol Sci. 2015 Oct;36(10):636-644. doi: 10.1016/j.tips.2015.06.007. Trends Pharmacol Sci. 2015. PMID: 26435209 Review.
-
The GABAB Receptor-Structure, Ligand Binding and Drug Development.Molecules. 2020 Jul 7;25(13):3093. doi: 10.3390/molecules25133093. Molecules. 2020. PMID: 32646032 Free PMC article. Review.
Cited by
-
Optical probes based on G protein-coupled receptors - added work or added value?Br J Pharmacol. 2016 Jan;173(2):255-66. doi: 10.1111/bph.13382. Epub 2015 Dec 19. Br J Pharmacol. 2016. PMID: 26562218 Free PMC article. Review.
-
Structure and functional interaction of the extracellular domain of human GABA(B) receptor GBR2.Nat Neurosci. 2012 Jun 3;15(7):970-8. doi: 10.1038/nn.3133. Nat Neurosci. 2012. PMID: 22660477 Free PMC article.
-
Structural Basis of GABAB Receptor Regulation and Signaling.Curr Top Behav Neurosci. 2022;52:19-37. doi: 10.1007/7854_2020_147. Curr Top Behav Neurosci. 2022. PMID: 32812202 Review.
-
Optogenetic reporters: Fluorescent protein-based genetically encoded indicators of signaling and metabolism in the brain.Prog Brain Res. 2012;196:235-63. doi: 10.1016/B978-0-444-59426-6.00012-4. Prog Brain Res. 2012. PMID: 22341329 Free PMC article. Review.
-
Activation of the γ-Aminobutyric Acid Type B (GABA(B)) Receptor by Agonists and Positive Allosteric Modulators.J Med Chem. 2015 Aug 27;58(16):6336-47. doi: 10.1021/jm5018913. Epub 2015 Apr 24. J Med Chem. 2015. PMID: 25856547 Free PMC article. Review.
References
-
- Pin J. P., Galvez T., Prézeau L. (2003) Pharmacol. Ther. 98, 325–354 - PubMed
-
- Gether U., Asmar F., Meinild A. K., Rasmussen S. G. (2002) Pharmacol. Toxicol. 91, 304–312 - PubMed
-
- Schwartz T. W., Frimurer T. M., Holst B., Rosenkilde M. M., Elling C. E. (2006) Annu. Rev. Pharmacol. Toxicol. 46, 481–519 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

