The dynein-tubulin motor powers active oscillations and amplification in the hearing organ of the mosquito
- PMID: 20129974
- PMCID: PMC2871864
- DOI: 10.1098/rspb.2009.2355
The dynein-tubulin motor powers active oscillations and amplification in the hearing organ of the mosquito
Erratum in
- Proc Biol Sci. 2011 Jun 7;278(1712):1760
Abstract
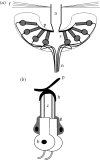
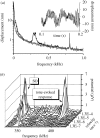
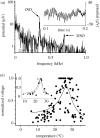
The design principles and specific proteins of the dynein-tubulin motor, which powers the flagella and cilia of eukaryotes, have been conserved throughout the evolution of life from algae to humans. Cilia and flagella can support both motile and sensory functions independently, or sometimes in parallel to each other. In this paper we show that this dual sensory-motile role of eukaryotic cilia is preserved in the most sensitive of all invertebrate hearing organs, the Johnston's organ of the mosquito. The Johnston's organ displays spontaneous oscillations, which have been identified as being a characteristic of amplification in the ears of mosquitoes and Drosophila. In the auditory organs of Drosophila and vertebrates, the molecular basis of amplification has been attributed to the gating and adaptation of the mechanoelectrical transducer channels themselves. On the basis of their temperature-dependence and sensitivity to colchicine, we attribute the molecular basis of spontaneous oscillations by the Johnston's organ of the mosquito Culex quinquefasciatus, to the dynein-tubulin motor of the ciliated sensillae. If, as has been claimed for insect and vertebrate hearing organs, spontaneous oscillations epitomize amplification, then in the mosquito ear, this process is independent of mechanotransduction.
Figures


References
-
- Albert J. T., Nadrowski B., Göpfert M. C.2007Mechanical signatures of transducer gating in the Drosophila ear. Curr. Biol. 17, 1000–1006 (doi:10.1016/j.cub.2007.05.004) - DOI - PubMed
-
- Anson M.1992Temperature-dependence and Arrhenius activation-energy of F-actin velocity generated in vitro by skeletal myosin. J. Mol. Biol. 224, 1029–1038 (doi:10.1016/0022-2836(92)90467-X) - DOI - PubMed
-
- Auger J., Serres C., Feneux D.1990Motion of individual human spermatozoa, both normal and lacking the outer dynein arms, during a continuous temperature rise. Cell Motil. Cytoskeleton 16, 22–32 (doi:10.1002/cm.970160105) - DOI - PubMed
-
- Bárány M.1967ATPase activity of myosin correlated with speed of muscle shortening. J. Gen. Physiol. 50, 197–218 (doi:10.1085/jgp.50.6.197) - DOI - PMC - PubMed
-
- Bohm K. J., Stracke R., Baum M., Zieren M., Unger E.2000Effect of temperature on kinesin-driven microtubule gliding and kinesin ATPase activity. FEBS Lett. 466, 59–62 (doi:10.1016/S0014-5793(99)01757-3) - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

