Origin of ultradian pulsatility in the hypothalamic-pituitary-adrenal axis
- PMID: 20129987
- PMCID: PMC2871854
- DOI: 10.1098/rspb.2009.2148
Origin of ultradian pulsatility in the hypothalamic-pituitary-adrenal axis
Abstract
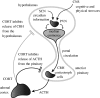
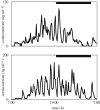
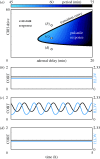
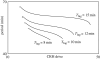
The hypothalamic-pituitary-adrenal (HPA) axis is a neuroendocrine system that regulates the circulating levels of vital glucocorticoid hormones. The activity of the HPA axis is characterized not only by a classic circadian rhythm, but also by an ultradian pattern of discrete pulsatile release of glucocorticoids. A number of psychiatric and metabolic diseases are associated with changes in glucocorticoid pulsatility, and it is now clear that glucocorticoid responsive genes respond to these rapid fluctuations in a biologically meaningful way. Theoretical modelling has enabled us to identify and explore potential mechanisms underlying the ultradian activity in this axis, which to date have not been identified successfully. We demonstrate that the combination of delay with feed-forward and feedback loops in the pituitary-adrenal system is sufficient to give rise to ultradian pulsatility in the absence of an ultradian source from a supra-pituitary site. Moreover, our model enables us to predict the different patterns of glucocorticoid release mediated by changes in hypophysial-portal corticotrophin-releasing hormone levels, with results that parallel our experimental in vivo data.
Figures


References
-
- Atkinson H. C., Wood S. A., Castrique E. S., Kershaw Y. M., Wiles C. C., Lightman S. L.2008Corticosteroids mediate fast feedback of the rat hypothalamic–pituitary–adrenal axis via the mineralocorticoid receptor. Am. J. Physiol. Endocrinol. Metab. 294, E1011–E1022 (doi:10.1152/ajpendo.00721.2007) - DOI - PubMed
-
- Belchetz P. E., Plant T. M., Nakai Y., Keogh E. J., Knobil E.1978Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science 202, 631–633 (doi:10.1126/science.100883) - DOI - PubMed
-
- Chrousos G. P.1995The hypothalamic–pituitary–adrenal axis and immune-mediated inflammation. N. Engl. J. Med. 332, 1351–1362 (doi:10.1056/NEJM199505183322008) - DOI - PubMed
-
- Clarke I. J.2002Two decades of measuring GnRH secretion. Reprod. Suppl. 59, 1–13 - PubMed
-
- Dallman M. F., Engeland W. C., Rose J. C., Wilkinson C. W., Shinsako J., Siedenburg F.1978Nycthemeral rhythm in adrenal responsiveness to ACTH. Am. J. Physiol. 235, R210–R218 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
