Direct evidence from single-cell analysis that human {alpha}-defensins block adenovirus uncoating to neutralize infection
- PMID: 20130047
- PMCID: PMC2849482
- DOI: 10.1128/JVI.02471-09
Direct evidence from single-cell analysis that human {alpha}-defensins block adenovirus uncoating to neutralize infection
Abstract
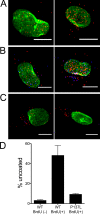
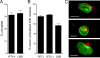
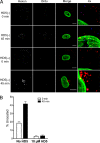
Human alpha-defensins are evolutionarily conserved effectors of the innate immune response with broadly acting antibacterial activity. Their role in antiviral immunity is less well understood. We previously showed that these antimicrobial peptides are potent inhibitors of human adenovirus infection. Based on biochemical studies and indirect evidence from confocal microscopy, we proposed that defensins bind to and stabilize the virus capsid and neutralize infection by preventing the release of the endosomalytic protein VI. To determine whether defensin action also restricts exposure of the viral genome, we developed a system to evaluate adenovirus uncoating during cell entry by monitoring the exposure of BrdU-labeled viral genomes. This assay allowed us to determine the kinetics of uncoating of virus particles in single cells. Using this assay, we now provide direct evidence that human alpha-defensins block adenovirus infection by preventing uncoating during cell entry.
Figures

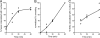
References
-
- Arnberg, N. 2009. Adenovirus receptors: implications for tropism, treatment and targeting. Rev. Med. Virol. 19:165-178. - PubMed
-
- Cerullo, V., M. P. Seiler, V. Mane, N. Brunetti-Pierri, C. Clarke, T. K. Bertin, J. R. Rodgers, and B. Lee. 2007. Toll-like receptor 9 triggers an innate immune response to helper-dependent adenoviral vectors. Mol. Ther. 15:378-385. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

