Killing of avian and Swine influenza virus by natural killer cells
- PMID: 20130050
- PMCID: PMC2849486
- DOI: 10.1128/JVI.02289-09
Killing of avian and Swine influenza virus by natural killer cells
Abstract
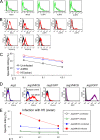
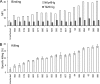
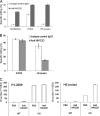
Today, global attention is focused on two influenza virus strains: the current pandemic strain, swine origin influenza virus (H1N1-2009), and the highly pathogenic avian influenza virus, H5N1. At present, the infection caused by the H1N1-2009 is moderate, with mortality rates of less <1%. In contrast, infection with the H5N1 virus resulted in high mortality rates, and ca. 60% of the infected patients succumb to the infection. Thus, one of the world greatest concerns is that the H5N1 virus will evolve to allow an efficient human infection and human-to-human transmission. Natural killer (NK) cells are one of the innate immune components playing an important role in fighting against influenza viruses. One of the major NK activating receptors involved in NK cell cytotoxicity is NKp46. We previously demonstrated that NKp46 recognizes the hemagglutinin proteins of B and A influenza virus strains. Whether NKp46 could also interact with H1N1-2009 virus or with the avian influenza virus is still unknown. We analyzed the immunological properties of both the avian and the H1N1-2009 influenza viruses. We show that NKp46 recognizes the hemagglutinins of H1N1-2009 and H5 and that this recognition leads to virus killing both in vitro and in vivo. However, importantly, while the swine H1-NKp46 interactions lead to the direct killing of the infected cells, the H5-NKp46 interactions were unable to elicit direct killing, probably because the NKp46 binding sites for these two viruses are different.
Figures



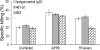
References
-
- Achdout, H., T. I. Arnon, G. Markel, T. Gonen-Gross, G. Katz, N. Lieberman, R. Gazit, A. Joseph, E. Kedar, and O. Mandelboim. 2003. Enhanced recognition of human NK receptors after influenza virus infection. J. Immunol. 171:915-923. - PubMed
-
- Arnon, T. I., H. Achdout, O. Levi, G. Markel, N. Saleh, G. Katz, R. Gazit, T. Gonen-Gross, J. Hanna, E. Nahari, A. Porgador, A. Honigman, B. Plachter, D. Mevorach, D. G. Wolf, and O. Mandelboim. 2005. Inhibition of the NKp30 activating receptor by pp65 of human cytomegalovirus. Nat. Immunol. 6:515-523. - PubMed
-
- Arnon, T. I., H. Achdout, N. Lieberman, R. Gazit, T. Gonen-Gross, G. Katz, A. Bar-Ilan, N. Bloushtain, M. Lev, A. Joseph, E. Kedar, A. Porgador, and O. Mandelboim. 2004. The mechanisms controlling the recognition of tumor- and virus-infected cells by NKp46. Blood 103:664-672. - PubMed
-
- Arnon, T. I., M. Lev, G. Katz, Y. Chernobrov, A. Porgador, and O. Mandelboim. 2001. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur. J. Immunol. 31:2680-2689. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

