The ORF3 protein of hepatitis E virus delays degradation of activated growth factor receptors by interacting with CIN85 and blocking formation of the Cbl-CIN85 complex
- PMID: 20130058
- PMCID: PMC2849493
- DOI: 10.1128/JVI.01994-09
The ORF3 protein of hepatitis E virus delays degradation of activated growth factor receptors by interacting with CIN85 and blocking formation of the Cbl-CIN85 complex
Abstract
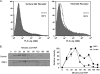
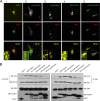
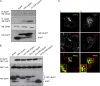
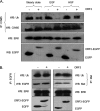
Hepatitis E virus (HEV) causes an acute self-limiting disease that is endemic in developing countries. Previous studies suggested that the ORF3 protein (pORF3) of HEV is required for infection in vivo and is likely to modulate the host response. Our previous work showed that pORF3 localizes to early and recycling endosomes and causes a delay in the postinternalization trafficking of epidermal growth factor receptor (EGFR) to late endosomes/lysosomes. Here we report that pORF3 also delays the trafficking and degradation of activated hepatocyte growth factor receptor (c-Met) and delineate the mechanistic details of these effects. A mutant ORF3 protein, which does not localize to endosomes, also showed similar effects on growth factor receptor trafficking, making this effect independent of the endosomal localization of pORF3. The ORF3 protein was found to interact with CIN85, a multidomain adaptor protein implicated in the Cbl-mediated downregulation of receptor tyrosine kinases. This interaction competed with the formation of the growth factor receptor-Cbl-CIN85 complex, resulting in the reduced ubiquitination of CIN85 and trafficking of the growth factor receptor complex toward late endosomes/lysosomes. We propose that through its effects on growth factor receptor trafficking, pORF3 prolongs endomembrane growth factor signaling and promotes cell survival to contribute positively to viral replication and pathogenesis.
Figures



Similar articles
-
The hepatitis E virus ORF3 protein modulates epidermal growth factor receptor trafficking, STAT3 translocation, and the acute-phase response.J Virol. 2008 Jul;82(14):7100-10. doi: 10.1128/JVI.00403-08. Epub 2008 Apr 30. J Virol. 2008. PMID: 18448545 Free PMC article.
-
The basic amino acids in the coiled-coil domain of CIN85 regulate its interaction with c-Cbl and phosphatidic acid during epidermal growth factor receptor (EGFR) endocytosis.BMC Biochem. 2014 Jul 8;15:13. doi: 10.1186/1471-2091-15-13. BMC Biochem. 2014. PMID: 25005938 Free PMC article.
-
Alix/AIP1 antagonizes epidermal growth factor receptor downregulation by the Cbl-SETA/CIN85 complex.Mol Cell Biol. 2004 Oct;24(20):8981-93. doi: 10.1128/MCB.24.20.8981-8993.2004. Mol Cell Biol. 2004. PMID: 15456872 Free PMC article.
-
[Ubiquitination-mediated degradation of epidermal growth factor receptor].Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2005 Feb;27(1):120-7. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2005. PMID: 15782507 Review. Chinese.
-
Met receptor dynamics and signalling.Curr Top Microbiol Immunol. 2004;286:21-44. doi: 10.1007/978-3-540-69494-6_2. Curr Top Microbiol Immunol. 2004. PMID: 15645709 Review.
Cited by
-
Three amino acid mutations (F51L, T59A, and S390L) in the capsid protein of the hepatitis E virus collectively contribute to virus attenuation.J Virol. 2011 Jun;85(11):5338-49. doi: 10.1128/JVI.02278-10. Epub 2011 Mar 30. J Virol. 2011. PMID: 21450834 Free PMC article.
-
Inhibition of CIN85-mediated invasion by a novel SH3 domain binding motif in the lysyl oxidase propeptide.PLoS One. 2013 Oct 22;8(10):e77288. doi: 10.1371/journal.pone.0077288. eCollection 2013. PLoS One. 2013. PMID: 24167568 Free PMC article.
-
Antiviral strategies for hepatitis E virus.Antiviral Res. 2014 Feb;102:106-18. doi: 10.1016/j.antiviral.2013.12.005. Epub 2013 Dec 25. Antiviral Res. 2014. PMID: 24374149 Free PMC article. Review.
-
Hepatitis E Virus Genome Structure and Replication Strategy.Cold Spring Harb Perspect Med. 2019 Jan 2;9(1):a031724. doi: 10.1101/cshperspect.a031724. Cold Spring Harb Perspect Med. 2019. PMID: 29530948 Free PMC article. Review.
-
Characteristics and Functions of HEV Proteins.Adv Exp Med Biol. 2023;1417:15-32. doi: 10.1007/978-981-99-1304-6_2. Adv Exp Med Biol. 2023. PMID: 37223856
References
-
- Amicone, L., F. M. Spagnoli, G. Spath, S. Giordano, C. Tommasini, S. Bernardini, V. De Luca, C. Della Rocca, M. C. Weiss, P. M. Comoglio, and M. Tripodi. 1997. Transgenic expression in the liver of truncated Met blocks apoptosis and permits immortalization of hepatocytes. EMBO J. 16:495-503. - PMC - PubMed
-
- Birchmeier, C., W. Birchmeier, E. Gherardi, and G. F. Vande Woude. 2003. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 4:915-925. - PubMed
-
- Brodin, L., P. Low, and O. Shupliakov. 2000. Sequential steps in clathrin-mediated synaptic vesicle endocytosis. Curr. Opin. Neurobiol. 10:312-320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

