A chimeric alphavirus replicon particle vaccine expressing the hemagglutinin and fusion proteins protects juvenile and infant rhesus macaques from measles
- PMID: 20130066
- PMCID: PMC2849488
- DOI: 10.1128/JVI.01566-09
A chimeric alphavirus replicon particle vaccine expressing the hemagglutinin and fusion proteins protects juvenile and infant rhesus macaques from measles
Abstract
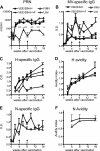
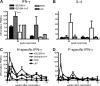
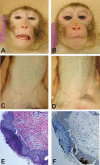
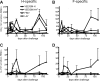
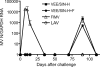
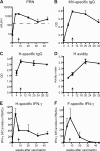
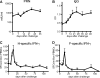
Measles remains a major cause of child mortality, in part due to an inability to vaccinate young infants with the current live attenuated virus vaccine (LAV). To explore new approaches to infant vaccination, chimeric Venezuelan equine encephalitis/Sindbis virus (VEE/SIN) replicon particles were used to express the hemagglutinin (H) and fusion (F) proteins of measles virus (MV). Juvenile rhesus macaques vaccinated intradermally with a single dose of VEE/SIN expressing H or H and F proteins (VEE/SIN-H or VEE/SIN-H+F, respectively) developed high titers of MV-specific neutralizing antibody and gamma-interferon (IFN-gamma)-producing T cells. Infant macaques vaccinated with two doses of VEE/SIN-H+F also developed neutralizing antibody and IFN-gamma-producing T cells. Control animals were vaccinated with LAV or with a formalin-inactivated measles vaccine (FIMV). Neutralizing antibody remained above the protective level for more than 1 year after vaccination with VEE/SIN-H, VEE/SIN-H+F, or LAV. When challenged with wild-type MV 12 to 17 months after vaccination, all vaccinated juvenile and infant monkeys vaccinated with VEE/SIN-H, VEE/SIN-H+F, and LAV were protected from rash and viremia, while FIMV-vaccinated monkeys were not. Antibody was boosted by challenge in all groups. T-cell responses to challenge were biphasic, with peaks at 7 to 25 days and at 90 to 110 days in all groups, except for the LAV group. Recrudescent T-cell activity coincided with the presence of MV RNA in peripheral blood mononuclear cells. We conclude that VEE/SIN expressing H or H and F induces durable immune responses that protect from measles and offers a promising new approach for measles vaccination. The viral and immunological factors associated with long-term control of MV replication require further investigation.
Figures

Similar articles
-
Vaxfectin adjuvant improves antibody responses of juvenile rhesus macaques to a DNA vaccine encoding the measles virus hemagglutinin and fusion proteins.J Virol. 2013 Jun;87(12):6560-8. doi: 10.1128/JVI.00635-13. Epub 2013 Apr 3. J Virol. 2013. PMID: 23552419 Free PMC article.
-
Modulation of disease, T cell responses, and measles virus clearance in monkeys vaccinated with H-encoding alphavirus replicon particles.Proc Natl Acad Sci U S A. 2005 Aug 16;102(33):11581-8. doi: 10.1073/pnas.0504592102. Epub 2005 Jul 21. Proc Natl Acad Sci U S A. 2005. PMID: 16037211 Free PMC article.
-
Poor immune responses of newborn rhesus macaques to measles virus DNA vaccines expressing the hemagglutinin and fusion glycoproteins.Clin Vaccine Immunol. 2013 Feb;20(2):205-10. doi: 10.1128/CVI.00394-12. Epub 2012 Dec 12. Clin Vaccine Immunol. 2013. PMID: 23239799 Free PMC article.
-
Measles virus.Hum Vaccin Immunother. 2015;11(1):21-6. doi: 10.4161/hv.34298. Epub 2014 Nov 1. Hum Vaccin Immunother. 2015. PMID: 25483511 Free PMC article. Review.
-
Measles Vaccine.Viral Immunol. 2018 Mar;31(2):86-95. doi: 10.1089/vim.2017.0143. Epub 2017 Dec 19. Viral Immunol. 2018. PMID: 29256824 Free PMC article. Review.
Cited by
-
TLR7/8 adjuvant overcomes newborn hyporesponsiveness to pneumococcal conjugate vaccine at birth.JCI Insight. 2017 Mar 23;2(6):e91020. doi: 10.1172/jci.insight.91020. JCI Insight. 2017. PMID: 28352660 Free PMC article.
-
Antibody-mediated protection against mucosal simian-human immunodeficiency virus challenge of macaques immunized with alphavirus replicon particles and boosted with trimeric envelope glycoprotein in MF59 adjuvant.J Virol. 2010 Jun;84(12):5975-85. doi: 10.1128/JVI.02533-09. Epub 2010 Apr 14. J Virol. 2010. PMID: 20392857 Free PMC article.
-
Vaxfectin adjuvant improves antibody responses of juvenile rhesus macaques to a DNA vaccine encoding the measles virus hemagglutinin and fusion proteins.J Virol. 2013 Jun;87(12):6560-8. doi: 10.1128/JVI.00635-13. Epub 2013 Apr 3. J Virol. 2013. PMID: 23552419 Free PMC article.
-
Development of nucleic acid vaccines: use of self-amplifying RNA in lipid nanoparticles.Int J Nanomedicine. 2014 Apr 10;9:1833-43. doi: 10.2147/IJN.S39810. eCollection 2014. Int J Nanomedicine. 2014. PMID: 24748793 Free PMC article. Review.
-
The Immune Response in Measles: Virus Control, Clearance and Protective Immunity.Viruses. 2016 Oct 12;8(10):282. doi: 10.3390/v8100282. Viruses. 2016. PMID: 27754341 Free PMC article. Review.
References
-
- Albrecht, P., F. A. Ennis, E. J. Saltzman, and S. Krugman. 1977. Persistence of maternal antibody in infants beyond 12 months: Mechanism of measles vaccine failure. J. Pediatr. 91:715-718. - PubMed
-
- Auwaerter, P. G., P. A. Rota, W. R. Elkins, R. J. Adams, T. DeLozier, Y. Shi, W. J. Bellini, B. R. Murphy, and D. E. Griffin. 1999. Measles virus infection in rhesus macaques: altered immune responses and comparison of the virulence of six different virus strains. J. Infect. Dis. 180:950-958. - PubMed
-
- Babaniyi, O. A., D. B. Parakoyi, B. A. Aiyedun, and M. A. Bello. 1995. Loss of maternally-acquired measles antibody during infancy in Ilorin, Nigeria. J. Trop. Pediatr. 41:115-117. - PubMed
-
- Beauverger, P., R. Buckland, and F. Wild. 1993. Establishment and characterisation of murine cells constitutively expressing the fusion, nucleoprotein and matrix proteins of measles virus. J. Virol. Methods 44:199-210. - PubMed
-
- Black, F. L. 1989. Measles active and passive immunity in a worldwide perspective. Prog. Med. Virol. 36:1-33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

