Manganese-induced trafficking and turnover of the cis-Golgi glycoprotein GPP130
- PMID: 20130081
- PMCID: PMC2847531
- DOI: 10.1091/mbc.e09-11-0985
Manganese-induced trafficking and turnover of the cis-Golgi glycoprotein GPP130
Abstract
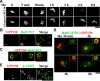
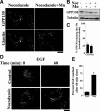
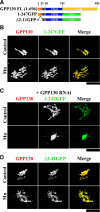
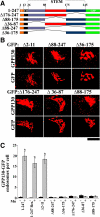
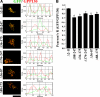
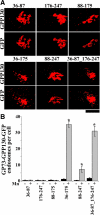
Manganese is an essential element that is also neurotoxic at elevated exposure. However, mechanisms regulating Mn homeostasis in mammalian cells are largely unknown. Because increases in cytosolic Mn induce rapid changes in the localization of proteins involved in regulating intracellular Mn concentrations in yeast, we were intrigued to discover that low concentrations of extracellular Mn induced rapid redistribution of the mammalian cis-Golgi glycoprotein Golgi phosphoprotein of 130 kDa (GPP130) to multivesicular bodies. GPP130 was subsequently degraded in lysosomes. The Mn-induced trafficking of GPP130 occurred from the Golgi via a Rab-7-dependent pathway and did not require its transit through the plasma membrane or early endosomes. Although the cytoplasmic domain of GPP130 was dispensable for its ability to respond to Mn, its lumenal stem domain was required and it had to be targeted to the cis-Golgi for the Mn response to occur. Remarkably, the stem domain was sufficient to confer Mn sensitivity to another cis-Golgi protein. Our results identify the stem domain of GPP130 as a novel Mn sensor in the Golgi lumen of mammalian cells.
Figures




Similar articles
-
Manganese-induced trafficking and turnover of GPP130 is mediated by sortilin.Mol Biol Cell. 2017 Sep 15;28(19):2569-2578. doi: 10.1091/mbc.E17-05-0326. Epub 2017 Aug 2. Mol Biol Cell. 2017. PMID: 28768823 Free PMC article.
-
Induced oligomerization targets Golgi proteins for degradation in lysosomes.Mol Biol Cell. 2015 Dec 1;26(24):4427-37. doi: 10.1091/mbc.E15-04-0207. Epub 2015 Oct 7. Mol Biol Cell. 2015. PMID: 26446839 Free PMC article.
-
Manganese induces oligomerization to promote down-regulation of the intracellular trafficking receptor used by Shiga toxin.Mol Biol Cell. 2014 Oct 1;25(19):3049-58. doi: 10.1091/mbc.E14-05-1003. Epub 2014 Jul 30. Mol Biol Cell. 2014. PMID: 25079690 Free PMC article.
-
Saccharomyces cerevisiae--a model organism for the studies on vacuolar transport.Acta Biochim Pol. 2001;48(4):1025-42. Acta Biochim Pol. 2001. PMID: 11995965 Review.
-
Targeting the Early Endosome-to-Golgi Transport of Shiga Toxins as a Therapeutic Strategy.Toxins (Basel). 2020 May 22;12(5):342. doi: 10.3390/toxins12050342. Toxins (Basel). 2020. PMID: 32456007 Free PMC article. Review.
Cited by
-
Manganese Is Essential for Neuronal Health.Annu Rev Nutr. 2015;35:71-108. doi: 10.1146/annurev-nutr-071714-034419. Epub 2015 May 13. Annu Rev Nutr. 2015. PMID: 25974698 Free PMC article. Review.
-
Deficiency in the manganese efflux transporter SLC30A10 induces severe hypothyroidism in mice.J Biol Chem. 2017 Jun 9;292(23):9760-9773. doi: 10.1074/jbc.M117.783605. Epub 2017 May 1. J Biol Chem. 2017. PMID: 28461334 Free PMC article.
-
Orchestration of SARS-CoV-2 Nsp4 and host cell ESCRT proteins induces morphological changes of the endoplasmic reticulum.Mol Biol Cell. 2025 Apr 1;36(4):ar40. doi: 10.1091/mbc.E24-12-0542. Epub 2025 Feb 12. Mol Biol Cell. 2025. PMID: 39937675 Free PMC article.
-
Golgi Metal Ion Homeostasis in Human Health and Diseases.Cells. 2022 Jan 15;11(2):289. doi: 10.3390/cells11020289. Cells. 2022. PMID: 35053405 Free PMC article. Review.
-
Functional analysis of SLC39A8 mutations and their implications for manganese deficiency and mitochondrial disorders.Sci Rep. 2018 Feb 16;8(1):3163. doi: 10.1038/s41598-018-21464-0. Sci Rep. 2018. PMID: 29453449 Free PMC article.
References
-
- Aschner M., Vrana K. E., Zheng W. Manganese uptake and distribution in the central nervous system (CNS) Neurotoxicology. 1999;20:173–180. - PubMed
-
- Behne M. J., Tu C. L., Aronchik I., Epstein E., Bench G., Bikle D. D., Pozzan T, Mauro T. M. Human keratinocyte ATP2C1 localizes to the Golgi and controls Golgi Ca2+ stores. J. Invest. Dermatol. 2003;121:688–694. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

