The Ero1alpha-PDI redox cycle regulates retro-translocation of cholera toxin
- PMID: 20130085
- PMCID: PMC2847533
- DOI: 10.1091/mbc.e09-09-0826
The Ero1alpha-PDI redox cycle regulates retro-translocation of cholera toxin
Abstract
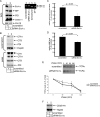
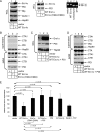
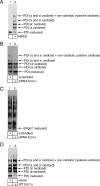
Cholera toxin (CT) is transported from the plasma membrane of host cells to the endoplasmic reticulum (ER) where the catalytic CTA1 subunit retro-translocates to the cytosol to induce toxicity. Our previous analyses demonstrated that the ER oxidoreductase protein disulfide isomerase (PDI) acts as a redox-dependent chaperone to unfold CTA1, a reaction postulated to initiate toxin retro-translocation. In its reduced state, PDI binds and unfolds CTA1; subsequent oxidation of PDI by Ero1alpha enables toxin release. Whether this in vitro model describes events in cells that control CTA1 retro-translocation is unknown. Here we show that down-regulation of Ero1alpha decreases retro-translocation of CTA1 by increasing reduced PDI and blocking efficient toxin release. Overexpression of Ero1alpha also attenuates CTA1 retro-translocation, an effect due to increased PDI oxidation, which prevents PDI from engaging the toxin effectively. Interestingly, Ero1alpha down-regulation increases interaction between PDI and Derlin-1, an ER membrane protein that is a component of the retro-translocation complex. These findings demonstrate that an appropriate Ero1alpha-PDI ratio is critical for regulating the binding-release cycle of CTA1 by PDI during retro-translocation, and implicate PDI's redox state in targeting it to the retro-translocon.
Figures


Similar articles
-
Derlin-1 facilitates the retro-translocation of cholera toxin.Mol Biol Cell. 2008 Mar;19(3):877-84. doi: 10.1091/mbc.e07-08-0755. Epub 2007 Dec 19. Mol Biol Cell. 2008. PMID: 18094046 Free PMC article.
-
The E3 ubiquitin ligases Hrd1 and gp78 bind to and promote cholera toxin retro-translocation.Mol Biol Cell. 2010 Jan 1;21(1):140-51. doi: 10.1091/mbc.e09-07-0586. Epub 2009 Oct 28. Mol Biol Cell. 2010. PMID: 19864457 Free PMC article.
-
Different interaction modes for protein-disulfide isomerase (PDI) as an efficient regulator and a specific substrate of endoplasmic reticulum oxidoreductin-1α (Ero1α).J Biol Chem. 2014 Nov 7;289(45):31188-99. doi: 10.1074/jbc.M114.602961. Epub 2014 Sep 25. J Biol Chem. 2014. PMID: 25258311 Free PMC article.
-
Cholera toxin: an intracellular journey into the cytosol by way of the endoplasmic reticulum.Toxins (Basel). 2010 Mar;2(3):310-25. doi: 10.3390/toxins2030310. Epub 2010 Mar 5. Toxins (Basel). 2010. PMID: 22069586 Free PMC article. Review.
-
Endoplasmic reticulum-dependent redox reactions control endoplasmic reticulum-associated degradation and pathogen entry.Antioxid Redox Signal. 2012 Apr 15;16(8):809-18. doi: 10.1089/ars.2011.4425. Epub 2012 Jan 30. Antioxid Redox Signal. 2012. PMID: 22142231 Free PMC article. Review.
Cited by
-
The delicate balance between secreted protein folding and endoplasmic reticulum-associated degradation in human physiology.Physiol Rev. 2012 Apr;92(2):537-76. doi: 10.1152/physrev.00027.2011. Physiol Rev. 2012. PMID: 22535891 Free PMC article. Review.
-
SOD1 aggregation in astrocytes following ischemia/reperfusion injury: a role of NO-mediated S-nitrosylation of protein disulfide isomerase (PDI).J Neuroinflammation. 2012 Oct 12;9:237. doi: 10.1186/1742-2094-9-237. J Neuroinflammation. 2012. PMID: 23061969 Free PMC article.
-
Stepwise assembly of fibrinogen is assisted by the endoplasmic reticulum lectin-chaperone system in HepG2 cells.PLoS One. 2013 Sep 10;8(9):e74580. doi: 10.1371/journal.pone.0074580. eCollection 2013. PLoS One. 2013. PMID: 24040290 Free PMC article.
-
A single native ganglioside GM1-binding site is sufficient for cholera toxin to bind to cells and complete the intoxication pathway.mBio. 2012 Oct 30;3(6):e00401-12. doi: 10.1128/mBio.00401-12. mBio. 2012. PMID: 23111873 Free PMC article.
-
Effects of ricin on primary pulmonary alveolar macrophages.J Int Med Res. 2019 Aug;47(8):3763-3777. doi: 10.1177/0300060519842959. Epub 2019 Jun 2. J Int Med Res. 2019. PMID: 31156015 Free PMC article.
References
-
- Appenzeller-Herzog C., Ellgaard L. In vivo reduction-oxidation state of protein disulfide isomerase: the two active sites independently occur in the reduced and oxidized forms. Antioxid. Redox Signal. 2008;10:55–64. - PubMed
-
- Bertoli G., Simmen T., Anelli T., Molteni S. N., Fesce R., Sitia R. Two conserved cysteine triads in human Ero1alpha cooperate for efficient disulfide bond formation in the endoplasmic reticulum. J. Biol. Chem. 2004;279:30047–30052. - PubMed
-
- Cabibbo A., Pagani M., Fabbri M., Rocchi M., Farmery M. R., Bulleid N. J., Sitia R. ERO1-L, a human protein that favors disulfide bond formation in the endoplasmic reticulum. J. Biol. Chem. 2000;275:4827–4833. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

