The Ero1alpha-PDI redox cycle regulates retro-translocation of cholera toxin
- PMID: 20130085
- PMCID: PMC2847533
- DOI: 10.1091/mbc.e09-09-0826
The Ero1alpha-PDI redox cycle regulates retro-translocation of cholera toxin
Abstract
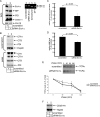
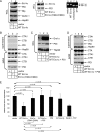
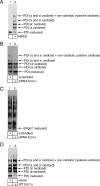
Cholera toxin (CT) is transported from the plasma membrane of host cells to the endoplasmic reticulum (ER) where the catalytic CTA1 subunit retro-translocates to the cytosol to induce toxicity. Our previous analyses demonstrated that the ER oxidoreductase protein disulfide isomerase (PDI) acts as a redox-dependent chaperone to unfold CTA1, a reaction postulated to initiate toxin retro-translocation. In its reduced state, PDI binds and unfolds CTA1; subsequent oxidation of PDI by Ero1alpha enables toxin release. Whether this in vitro model describes events in cells that control CTA1 retro-translocation is unknown. Here we show that down-regulation of Ero1alpha decreases retro-translocation of CTA1 by increasing reduced PDI and blocking efficient toxin release. Overexpression of Ero1alpha also attenuates CTA1 retro-translocation, an effect due to increased PDI oxidation, which prevents PDI from engaging the toxin effectively. Interestingly, Ero1alpha down-regulation increases interaction between PDI and Derlin-1, an ER membrane protein that is a component of the retro-translocation complex. These findings demonstrate that an appropriate Ero1alpha-PDI ratio is critical for regulating the binding-release cycle of CTA1 by PDI during retro-translocation, and implicate PDI's redox state in targeting it to the retro-translocon.
Figures


References
-
- Appenzeller-Herzog C., Ellgaard L. In vivo reduction-oxidation state of protein disulfide isomerase: the two active sites independently occur in the reduced and oxidized forms. Antioxid. Redox Signal. 2008;10:55–64. - PubMed
-
- Bertoli G., Simmen T., Anelli T., Molteni S. N., Fesce R., Sitia R. Two conserved cysteine triads in human Ero1alpha cooperate for efficient disulfide bond formation in the endoplasmic reticulum. J. Biol. Chem. 2004;279:30047–30052. - PubMed
-
- Cabibbo A., Pagani M., Fabbri M., Rocchi M., Farmery M. R., Bulleid N. J., Sitia R. ERO1-L, a human protein that favors disulfide bond formation in the endoplasmic reticulum. J. Biol. Chem. 2000;275:4827–4833. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

