Regulation of renal proximal tubule Na-K-ATPase by prostaglandins
- PMID: 20130120
- PMCID: PMC2867409
- DOI: 10.1152/ajprenal.00467.2009
Regulation of renal proximal tubule Na-K-ATPase by prostaglandins
Abstract
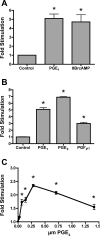
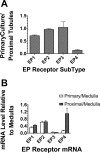
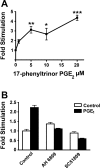
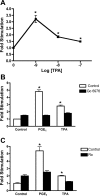
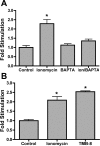
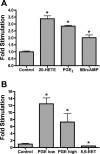
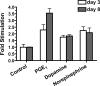
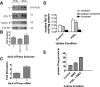
Prostaglandins (PGs) play a number of roles in the kidney, including regulation of salt and water reabsorption. In this report, evidence was obtained for stimulatory effects of PGs on Na-K-ATPase in primary cultures of rabbit renal proximal tubule (RPT) cells. The results of our real-time PCR studies indicate that in primary RPTs the effects of PGE(2), the major renal PG, are mediated by four classes of PGE (EP) receptors. The role of these EP receptors in the regulation of Na-K-ATPase was examined at the transcriptional level. Na-K-ATPase consists of a catalytic α-subunit encoded by the ATP1A1 gene, as well as a β-subunit encoded by the ATP1B1 gene. Transient transfection studies conducted with pHβ1-1141 Luc, a human ATP1B1 promoter/luciferase construct, indicate that both PGE(1) and PGE(2) are stimulatory. The evidence for the involvement of both the cAMP and Ca(2+) signaling pathways includes the inhibitory effects of the myristolylated PKA inhibitor PKI, the adenylate cyclase (AC) inhibitor SQ22536, and the PKC inhibitors Gö 6976 and Ro-32-0432 on the PGE(1) stimulation. Other effectors that similarly act through cAMP and PKC were also stimulatory to transcription, including norepinephrine and dopamine. In addition to its effects on transcription, a chronic incubation with PGE(1) was observed to result in an increase in Na-K-ATPase mRNA levels as well as an increase in Na-K-ATPase activity. An acute stimulatory effect of PGE(1) on Na-K-ATPase was observed and was associated with an increase in the level of Na-K-ATPase in the basolateral membrane.
Figures


References
-
- Alavi N, Lianos EA, Bentzel CJ. Prostaglandin and thromboxane synthesis by highly enriched rabbit proximal tubular cells in culture. J Lab Clin Med 110: 338–345, 1987 - PubMed
-
- Aperia A, Holtbèack U, Syrâen ML, Svensson LB, Fryckstedt J, Greengard P. Activation/deactivation of renal Na+, K+-ATPase: a final common pathway for regulation of natriuresis. FASEB J 8: 436–439, 1994 - PubMed
-
- Aperia AC. Intrarenal dopamine: a key signal in the interactive regulation of sodium metabolism. Annu Rev Physiol 62: 621–647, 2000 - PubMed
-
- Baginski ES, Foa PP, Zak B. Microdetermination of inorganic phosphate, phospholipids, and total phosphate in biologic materials. Clin Chem 13: 326–332, 1967 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

