Optimization and application of a multiplex bead-based assay to quantify serotype-specific IgG against Streptococcus pneumoniae polysaccharides: response to the booster vaccine after immunization with the pneumococcal 7-valent conjugate vaccine
- PMID: 20130129
- PMCID: PMC2849341
- DOI: 10.1128/CVI.00408-09
Optimization and application of a multiplex bead-based assay to quantify serotype-specific IgG against Streptococcus pneumoniae polysaccharides: response to the booster vaccine after immunization with the pneumococcal 7-valent conjugate vaccine
Abstract
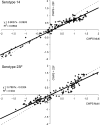
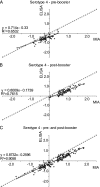
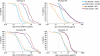
We describe the optimization and application of a multiplex bead-based assay (Luminex) to quantify antibodies against polysaccharides of 13 pneumococcal serotypes. In the optimized multiplex immunoassay (MIA), intravenous immune globulin was introduced as an in-house reference serum, and nonspecific reacting antibodies were adsorbed with the commercial product pneumococcal C polysaccharides Multi. The antibody concentrations were assessed in 188 serum samples obtained pre- and post-booster vaccination at 11 months after administration of a primary series of the pneumococcal seven-valent conjugate vaccine (PCV-7) at 2, 3, and 4 months of age. The results of the MIA were compared with those of the ELISA for the serotypes included in the seven-valent conjugated polysaccharide vaccine and for a non-vaccine serotype, serotype 6A. The geometric mean concentrations of the antibodies determined by MIA were slightly higher than those determined by ELISA. The correlations between the assays were good, with R(2) values ranging from 0.84 to 0.91 for all serotypes except serotype 19F, for which R(2) was 0.70. The concentrations of antibody against serotype 6A increased after the administration of PCV-7 due to cross-reactivity with serotype 6B. The differences between the results obtained by ELISA and MIA suggest that the internationally established protective threshold of 0.35 microg/ml should be reevaluated for use in the MIA and may need to be amended separately for each serotype.
Figures




Similar articles
-
Evaluation of a Validated Luminex-Based Multiplex Immunoassay for Measuring Immunoglobulin G Antibodies in Serum to Pneumococcal Capsular Polysaccharides.mSphere. 2018 Aug 8;3(4):e00127-18. doi: 10.1128/mSphere.00127-18. mSphere. 2018. PMID: 30089644 Free PMC article.
-
Development and Validation of 13-plex Luminex-Based Assay for Measuring Human Serum Antibodies to Streptococcus pneumoniae Capsular Polysaccharides.mSphere. 2018 Aug 8;3(4):e00128-18. doi: 10.1128/mSphere.00128-18. mSphere. 2018. PMID: 30089645 Free PMC article.
-
Immune response to pneumococcal conjugate vaccination in asplenic individuals.Hum Vaccin. 2009 Feb;5(2):85-91. doi: 10.4161/hv.5.2.6557. Epub 2009 Feb 7. Hum Vaccin. 2009. PMID: 18758242
-
Immunogenicity differences of a 15-valent pneumococcal polysaccharide conjugate vaccine (PCV15) based on vaccine dose, route of immunization and mouse strain.Vaccine. 2017 Feb 7;35(6):865-872. doi: 10.1016/j.vaccine.2016.12.055. Epub 2017 Jan 10. Vaccine. 2017. PMID: 28087148 Review.
-
Age-related immune response to pneumococcal polysaccharide vaccination: lessons for the clinic.Expert Rev Vaccines. 2015 Jan;14(1):85-97. doi: 10.1586/14760584.2015.963058. Epub 2014 Oct 1. Expert Rev Vaccines. 2015. PMID: 25269650 Review.
Cited by
-
Serotype-specific anti-pneumococcal IgG and immune competence: critical differences in interpretation criteria when different methods are used.J Clin Immunol. 2013 Feb;33(2):335-41. doi: 10.1007/s10875-012-9806-9. Epub 2012 Sep 30. J Clin Immunol. 2013. PMID: 23054341
-
Direct Comparison of Immunogenicity Induced by 10- or 13-Valent Pneumococcal Conjugate Vaccine around the 11-Month Booster in Dutch Infants.PLoS One. 2015 Dec 10;10(12):e0144739. doi: 10.1371/journal.pone.0144739. eCollection 2015. PLoS One. 2015. PMID: 26658902 Free PMC article. Clinical Trial.
-
Age-related decline in IgM responses associate with reduced opsonophagocytic activity following PCV13 vaccination.NPJ Vaccines. 2025 May 14;10(1):95. doi: 10.1038/s41541-025-01152-7. NPJ Vaccines. 2025. PMID: 40369006 Free PMC article.
-
Development of a bead-based multiplex immunoassay for simultaneous quantitative detection of IgG serum antibodies against measles, mumps, rubella, and varicella-zoster virus.Clin Vaccine Immunol. 2012 Mar;19(3):396-400. doi: 10.1128/CVI.05537-11. Epub 2012 Jan 11. Clin Vaccine Immunol. 2012. PMID: 22237896 Free PMC article.
-
Correlation of Vaccine Responses.Front Immunol. 2021 Apr 2;12:646677. doi: 10.3389/fimmu.2021.646677. eCollection 2021. Front Immunol. 2021. PMID: 33868282 Free PMC article.
References
-
- Berbers, G. A., and N. Jones. 2008. Serological surveillance of the effect of the changes to pertussis vaccines in the NIP from 2004 till 2008. Switch from whole cell to acellular vaccine in children of 1 year of age (ISRCTN97785537). RIVM report. National Institute for Public Health and the Environment, Bilthoven, Netherlands.
-
- Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, and K. Edwards. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr. Infect. Dis. J. 19:187-195. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

