An inactivated Vero cell-grown Japanese encephalitis vaccine formulated with Advax, a novel inulin-based adjuvant, induces protective neutralizing antibody against homologous and heterologous flaviviruses
- PMID: 20130134
- PMCID: PMC2888167
- DOI: 10.1099/vir.0.019190-0
An inactivated Vero cell-grown Japanese encephalitis vaccine formulated with Advax, a novel inulin-based adjuvant, induces protective neutralizing antibody against homologous and heterologous flaviviruses
Abstract
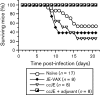
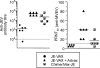
Advax is a polysaccharide-based adjuvant that potently stimulates vaccine immunogenicity without the increased reactogenicity seen with other adjuvants. This study investigated the immunogenicity of a novel Advax-adjuvanted Vero cell culture candidate vaccine against Japanese encephalitis virus (JEV) in mice and horses. The results showed that, in mice, a two-immunization, low-dose (50 ng JEV antigen) regimen with adjuvanted vaccine produced solid neutralizing immunity comparable to that elicited with live ChimeriVax-JE immunization and superior to that elicited with tenfold higher doses of a traditional non-adjuvanted JEV vaccine (JE-VAX; Biken Institute) or a newly approved alum-adjuvanted vaccine (Jespect; Novartis). Mice vaccinated with the Advax-adjuvanted, but not the unadjuvanted vaccine, were protected against live JEV challenge. Equine immunizations against JEV with Advax-formulated vaccine similarly showed enhanced vaccine immunogenicity, confirming that the adjuvant effects of Advax are not restricted to rodent models. Advax-adjuvanted JEV vaccine elicited a balanced T-helper 1 (Th1)/Th2 immune response against JEV with protective levels of cross-neutralizing antibody against other viruses belonging to the JEV serocomplex, including Murray Valley encephalitis virus (MVEV). The adjuvanted JEV vaccine was well tolerated with minimal reactogenicity and no systemic toxicity in immunized animals. The cessation of manufacture of traditional mouse brain-derived unadjuvanted JEV vaccine in Japan has resulted in a JEV vaccine shortage internationally. There is also an ongoing lack of human vaccines against other JEV serocomplex flaviviruses, such as MVEV, making this adjuvanted, cell culture-grown JEV vaccine a promising candidate to address both needs with one vaccine.
Figures
Similar articles
-
Safety and immunogenicity of a delta inulin-adjuvanted inactivated Japanese encephalitis virus vaccine in pregnant mares and foals.Vet Res. 2014 Dec 17;45(1):130. doi: 10.1186/s13567-014-0130-7. Vet Res. 2014. PMID: 25516480 Free PMC article.
-
An inactivated cell culture Japanese encephalitis vaccine (JE-ADVAX) formulated with delta inulin adjuvant provides robust heterologous protection against West Nile encephalitis via cross-protective memory B cells and neutralizing antibody.J Virol. 2013 Sep;87(18):10324-33. doi: 10.1128/JVI.00480-13. Epub 2013 Jul 17. J Virol. 2013. PMID: 23864620 Free PMC article.
-
JE-ADVAX vaccine protection against Japanese encephalitis virus mediated by memory B cells in the absence of CD8(+) T cells and pre-exposure neutralizing antibody.J Virol. 2013 Apr;87(8):4395-402. doi: 10.1128/JVI.03144-12. Epub 2013 Feb 6. J Virol. 2013. PMID: 23388724 Free PMC article.
-
Comparing the immunogenicity and safety of 3 Japanese encephalitis vaccines in Asia-Pacific area: A systematic review and meta-analysis.Hum Vaccin Immunother. 2015;11(6):1418-25. doi: 10.1080/21645515.2015.1011996. Hum Vaccin Immunother. 2015. PMID: 25915588 Free PMC article.
-
Product review on the JE vaccine IXIARO.Hum Vaccin Immunother. 2015;11(2):411-20. doi: 10.4161/21645515.2014.983412. Hum Vaccin Immunother. 2015. PMID: 25621812 Free PMC article. Review.
Cited by
-
The chemokine receptor CCR5, a therapeutic target for HIV/AIDS antagonists, is critical for recovery in a mouse model of Japanese encephalitis.PLoS One. 2012;7(9):e44834. doi: 10.1371/journal.pone.0044834. Epub 2012 Sep 21. PLoS One. 2012. PMID: 23028638 Free PMC article.
-
Comparison of JEV neutralization assay using pseudotyped JEV with the conventional plaque-reduction neutralization test.J Microbiol. 2014 May;52(5):435-40. doi: 10.1007/s12275-014-3529-y. Epub 2014 Mar 7. J Microbiol. 2014. PMID: 24610332 Free PMC article.
-
Safety and immunogenicity of a delta inulin-adjuvanted inactivated Japanese encephalitis virus vaccine in pregnant mares and foals.Vet Res. 2014 Dec 17;45(1):130. doi: 10.1186/s13567-014-0130-7. Vet Res. 2014. PMID: 25516480 Free PMC article.
-
Advax™, a novel microcrystalline polysaccharide particle engineered from delta inulin, provides robust adjuvant potency together with tolerability and safety.Vaccine. 2015 Nov 4;33(44):5920-6. doi: 10.1016/j.vaccine.2015.09.030. Epub 2015 Sep 25. Vaccine. 2015. PMID: 26407920 Free PMC article. Review.
-
Advax™, a polysaccharide adjuvant derived from delta inulin, provides improved influenza vaccine protection through broad-based enhancement of adaptive immune responses.Vaccine. 2012 Aug 3;30(36):5373-81. doi: 10.1016/j.vaccine.2012.06.021. Epub 2012 Jun 20. Vaccine. 2012. PMID: 22728225 Free PMC article.
References
-
- Appaiahgari, M. B. & Vrati, S. (2004). Immunogenicity and protective efficacy in mice of a formaldehyde-inactivated Indian strain of Japanese encephalitis virus grown in Vero cells. Vaccine 22, 3669–3675. - PubMed
-
- Barrett, A. D. & Gould, E. A. (1986). Comparison of neurovirulence of different strains of yellow fever virus in mice. J Gen Virol 67, 631–637. - PubMed
-
- Beasley, D. W., Lewthwaite, P. & Solomon, T. (2008). Current use and development of vaccines for Japanese encephalitis. Expert Opin Biol Ther 8, 95–106. - PubMed
-
- Broom, A. K., Wallace, M. J., Mackenzie, J. S., Smith, D. W. & Hall, R. A. (2000). Immunization with gamma globulin of Murray Valley encephalitis virus and with an inactivated Japanese encephalitis virus vaccine as prophylaxis against Australian encephalitis: evaluation in a mouse model. J Med Virol 61, 259–265. - PubMed
-
- Chu, J. H., Chiang, C. C. & Ng, M. L. (2007). Immunization of flavivirus West Nile recombinant envelope domain III protein induced specific immune response and protection against West Nile virus infection. J Immunol 178, 2699–2705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

