Matrix elasticity, cytoskeletal forces and physics of the nucleus: how deeply do cells 'feel' outside and in?
- PMID: 20130138
- PMCID: PMC2816180
- DOI: 10.1242/jcs.041186
Matrix elasticity, cytoskeletal forces and physics of the nucleus: how deeply do cells 'feel' outside and in?
Abstract
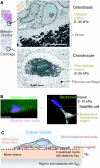
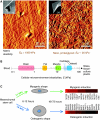
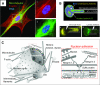
Cellular organization within a multicellular organism requires that a cell assess its relative location, taking in multiple cues from its microenvironment. Given that the extracellular matrix (ECM) consists of the most abundant proteins in animals and contributes both structure and elasticity to tissues, ECM probably provides key physical cues to cells. In vivo, in the vicinity of many tissue cell types, fibrous characteristics of the ECM are less discernible than the measurably distinct elasticity that characterizes different tissue microenvironments. As a cell engages matrix and actively probes, it senses the local elastic resistance of the ECM and nearby cells via their deformation, and--similar to the proverbial princess who feels a pea placed many mattresses below--the cell seems to possess feedback and recognition mechanisms that establish how far it can feel. Recent experimental findings and computational modeling of cell and matrix mechanics lend insight into the subcellular range of sensitivity. Continuity of deformation from the matrix into the cell and further into the cytoskeleton-caged and -linked nucleus also supports the existence of mechanisms that direct processes such as gene expression in the differentiation of stem cells. Ultimately, cells feel the difference between stiff or soft and thick or thin surroundings, regardless of whether or not they are of royal descent.
Figures


Similar articles
-
Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics.Biophys J. 2006 May 15;90(10):3762-73. doi: 10.1529/biophysj.105.071506. Epub 2006 Feb 24. Biophys J. 2006. PMID: 16500961 Free PMC article.
-
How Nucleus Mechanics and ECM Microstructure Influence the Invasion of Single Cells and Multicellular Aggregates.Bull Math Biol. 2018 May;80(5):1017-1045. doi: 10.1007/s11538-017-0262-9. Epub 2017 Apr 13. Bull Math Biol. 2018. PMID: 28409417
-
Measuring nucleus mechanics within a living multicellular organism: Physical decoupling and attenuated recovery rate are physiological protective mechanisms of the cell nucleus under high mechanical load.Mol Biol Cell. 2020 Aug 1;31(17):1943-1950. doi: 10.1091/mbc.E20-01-0085. Epub 2020 Jun 17. Mol Biol Cell. 2020. PMID: 32583745 Free PMC article.
-
Conformational changes and signaling in cell and matrix physics.Curr Biol. 2009 Sep 15;19(17):R781-9. doi: 10.1016/j.cub.2009.06.054. Curr Biol. 2009. PMID: 19906580 Free PMC article. Review.
-
The matrix environmental and cell mechanical properties regulate cell migration and contribute to the invasive phenotype of cancer cells.Rep Prog Phys. 2019 Jun;82(6):064602. doi: 10.1088/1361-6633/ab1628. Epub 2019 Apr 4. Rep Prog Phys. 2019. PMID: 30947151 Review.
Cited by
-
Biomaterials for Three-Dimensional Cell Culture: From Applications in Oncology to Nanotechnology.Nanomaterials (Basel). 2021 Feb 13;11(2):481. doi: 10.3390/nano11020481. Nanomaterials (Basel). 2021. PMID: 33668665 Free PMC article. Review.
-
Computational estimates of mechanical constraints on cell migration through the extracellular matrix.PLoS Comput Biol. 2020 Aug 27;16(8):e1008160. doi: 10.1371/journal.pcbi.1008160. eCollection 2020 Aug. PLoS Comput Biol. 2020. PMID: 32853248 Free PMC article.
-
3D Cultivation Techniques for Primary Human Hepatocytes.Microarrays (Basel). 2015 Feb 16;4(1):64-83. doi: 10.3390/microarrays4010064. Microarrays (Basel). 2015. PMID: 27600213 Free PMC article. Review.
-
Exploring gellan gum-based hydrogels for regenerating human embryonic stem cells in age-related macular degeneration therapy: A literature review.Regen Ther. 2024 Jun 10;26:235-250. doi: 10.1016/j.reth.2024.05.018. eCollection 2024 Jun. Regen Ther. 2024. PMID: 38966602 Free PMC article. Review.
-
Cell infiltrative hydrogel fibrous scaffolds for accelerated wound healing.Acta Biomater. 2017 Feb;49:66-77. doi: 10.1016/j.actbio.2016.11.017. Epub 2016 Nov 5. Acta Biomater. 2017. PMID: 27826004 Free PMC article.
References
-
- Alenghat F. J., Ingber D. E. (2002). Mechanotransduction: all signals point to cytoskeleton, matrix, and integrins. Science STKE 119, pe6 - PubMed
-
- Alexopoulos L. G., Williams G. M., Upton M. L., Setton L. A., Guilak F. (2005). Osteoarthritic changes in the biphasic mechanical properties of the chondrocyte pericellular matrix in articular cartilage. J. Biomech. 38, 509-517 - PubMed
-
- An S. S., Fabry B., Mellema M., Bursac P., Gerthoffer W. T., Kayyali U. S., Gaestel M., Shore S. A., Fredberg J. J. (2004). Role of heat shock protein 27 in cytoskeletal remodeling of the airway smooth muscle cell. J. Appl. Physiol. 96, 1701-1713 - PubMed
-
- Andrades J. A., Santamaria J. A., Nimni M. E., Becerra J. (2001). Selection and amplification of a bone marrow cell population and its induction to the chondro-osteogenic lineage by rhOP-1: an in vitro and in vivo study. Int. J. Dev. Biol. 45, 689-693 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

