Cognitive loss in zinc transporter-3 knock-out mice: a phenocopy for the synaptic and memory deficits of Alzheimer's disease?
- PMID: 20130173
- PMCID: PMC6633978
- DOI: 10.1523/JNEUROSCI.5255-09.2010
Cognitive loss in zinc transporter-3 knock-out mice: a phenocopy for the synaptic and memory deficits of Alzheimer's disease?
Abstract
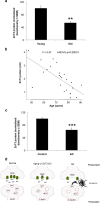
Zinc transporter-3 (ZnT3) protein controls synaptic vesicular Zn(2+) levels, which is predicted to regulate normal cognitive function. Surprisingly, previous studies found that 6- to 10-week-old ZnT3 knock-out (KO) mice did not show impairment in the Morris water maze. We hypothesized that older ZnT3 KO animals would display a cognitive phenotype. Here, we report that ZnT3 KO mice exhibit age-dependent deficits in learning and memory that are manifest at 6 months but not at 3 months of age. These deficits are associated with significant alterations in key hippocampal proteins involved in learning and memory, as assessed by Western blot. These include decreased levels of the presynaptic protein SNAP25 (-46%; p < 0.01); the postsynaptic protein PSD95 (-37%; p < 0.01); the glutamate receptors AMPAR (-34%; p < 0.01), NMDAR2a (-64%; p < 0.001), and NMDAR2b (-49%; p < 0.05); the surrogate marker of neurogenesis doublecortin (-31%; p < 0.001); and elements of the BDNF pathway, pro-BDNF (-30%; p < 0.05) and TrkB (-22%; p < 0.01). In addition, there is a concomitant decrease in neuronal spine density (-6%; p < 0.05). We also found that cortical ZnT3 levels fall with age in wild-type mice (-50%; p < 0.01) in healthy older humans (ages, 48-91 years; r(2) = 0.47; p = 0.00019) and particularly in Alzheimer's disease (AD) (-36%; p < 0.0001). Thus, age-dependent loss of transsynaptic Zn(2+) movement leads to cognitive loss, and since extracellular beta-amyloid is aggregated by and traps this pool of Zn(2+), the genetic ablation of ZnT3 may represent a phenocopy for the synaptic and memory deficits of AD.
Figures


References
-
- Adlard PA, Cherny RA, Finkelstein DI, Gautier E, Robb E, Cortes M, Volitakis I, Liu X, Smith JP, Perez K, Laughton K, Li QX, Charman SA, Nicolazzo JA, Wilkins S, Deleva K, Lynch T, Kok G, Ritchie CW, Tanzi RE, et al. Rapid restoration of cognition in Alzheimer's transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial Abeta. Neuron. 2008;59:43–55. - PubMed
-
- Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–276. - PubMed
-
- Chowanadisai W, Kelleher SL, Lönnerdal B. Maternal zinc deficiency reduces NMDA receptor expression in neonatal rat brain, which persists into early adulthood. J Neurochem. 2005;94:510–519. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
