ERK1/2 mitogen-activated protein kinase phosphorylates sodium channel Na(v)1.7 and alters its gating properties
- PMID: 20130174
- PMCID: PMC6633991
- DOI: 10.1523/JNEUROSCI.4872-09.2010
ERK1/2 mitogen-activated protein kinase phosphorylates sodium channel Na(v)1.7 and alters its gating properties
Abstract
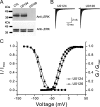
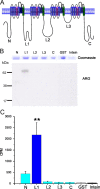
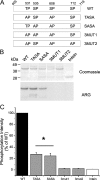
Na(v)1.7 sodium channels can amplify weak stimuli in neurons and act as threshold channels for firing action potentials. Neurotrophic factors and pro-nociceptive cytokines that are released during development and under pathological conditions activate mitogen-activated protein kinases (MAPKs). Previous studies have shown that MAPKs can transduce developmental or pathological signals by regulating transcription factors that initiate a gene expression response, a long-term effect, and directly modulate neuronal ion channels including sodium channels, thus acutely regulating dorsal root ganglion (DRG) neuron excitability. For example, neurotrophic growth factor activates (phosphorylates) ERK1/2 MAPK (pERK1/2) in DRG neurons, an effect that has been implicated in injury-induced hyperalgesia. However, the acute effects of pERK1/2 on sodium channels are not known. We have shown previously that activated p38 MAPK (pp38) directly phosphorylates Na(v)1.6 and Na(v)1.8 sodium channels and regulates their current densities without altering their gating properties. We now report that acute inhibition of pERK1/2 regulates resting membrane potential and firing properties of DRG neurons. We also show that pERK1 phosphorylates specific residues within L1 of Na(v)1.7, inhibition of pERK1/2 causes a depolarizing shift of activation and fast inactivation of Na(v)1.7 without altering current density, and mutation of these L1 phosphoacceptor sites abrogates the effect of pERK1/2 on this channel. Together, these data are consistent with direct phosphorylation and modulation of Na(v)1.7 by pERK1/2, which unlike the modulation of Na(v)1.6 and Na(v)1.8 by pp38, regulates gating properties of this channel but not its current density and contributes to the effects of MAPKs on DRG neuron excitability.
Figures

Similar articles
-
Phosphorylation of sodium channel Na(v)1.8 by p38 mitogen-activated protein kinase increases current density in dorsal root ganglion neurons.J Neurosci. 2008 Mar 19;28(12):3190-201. doi: 10.1523/JNEUROSCI.4403-07.2008. J Neurosci. 2008. PMID: 18354022 Free PMC article.
-
Glycosylation alters steady-state inactivation of sodium channel Nav1.9/NaN in dorsal root ganglion neurons and is developmentally regulated.J Neurosci. 2001 Dec 15;21(24):9629-37. doi: 10.1523/JNEUROSCI.21-24-09629.2001. J Neurosci. 2001. PMID: 11739573 Free PMC article.
-
Gating properties of Na(v)1.7 and Na(v)1.8 peripheral nerve sodium channels.J Neurosci. 2001 Oct 15;21(20):7909-18. doi: 10.1523/JNEUROSCI.21-20-07909.2001. J Neurosci. 2001. PMID: 11588164 Free PMC article.
-
GDNF and NGF reverse changes in repriming of TTX-sensitive Na(+) currents following axotomy of dorsal root ganglion neurons.J Neurophysiol. 2002 Aug;88(2):650-8. doi: 10.1152/jn.2002.88.2.650. J Neurophysiol. 2002. PMID: 12163518
-
Regulation/modulation of sensory neuron sodium channels.Handb Exp Pharmacol. 2014;221:111-35. doi: 10.1007/978-3-642-41588-3_6. Handb Exp Pharmacol. 2014. PMID: 24737234 Review.
Cited by
-
Sodium channel Na(v)1.7 is essential for lowering heat pain threshold after burn injury.J Neurosci. 2012 Aug 8;32(32):10819-32. doi: 10.1523/JNEUROSCI.0304-12.2012. J Neurosci. 2012. PMID: 22875917 Free PMC article.
-
Targeting voltage gated sodium channels NaV1.7, Na V1.8, and Na V1.9 for treatment of pathological cough.Lung. 2014 Feb;192(1):15-20. doi: 10.1007/s00408-013-9533-x. Epub 2013 Nov 24. Lung. 2014. PMID: 24272479 Free PMC article. Review.
-
Nav1.7 is phosphorylated by Fyn tyrosine kinase which modulates channel expression and gating in a cell type-dependent manner.Mol Pain. 2018 Jan-Dec;14:1744806918782229. doi: 10.1177/1744806918782229. Epub 2018 May 23. Mol Pain. 2018. PMID: 29790812 Free PMC article.
-
AMPK: An emerging target for modification of injury-induced pain plasticity.Neurosci Lett. 2013 Dec 17;557 Pt A(0 0):9-18. doi: 10.1016/j.neulet.2013.06.060. Epub 2013 Jul 3. Neurosci Lett. 2013. PMID: 23831352 Free PMC article. Review.
-
Nerve Growth Factor Signaling and Its Contribution to Pain.J Pain Res. 2020 May 26;13:1223-1241. doi: 10.2147/JPR.S247472. eCollection 2020. J Pain Res. 2020. PMID: 32547184 Free PMC article. Review.
References
-
- Adams JP, Anderson AE, Varga AW, Dineley KT, Cook RG, Pfaffinger PJ, Sweatt JD. The A-type potassium channel Kv4.2 is a substrate for the mitogen-activated protein kinase ERK. J Neurochem. 2000;75:2277–2287. - PubMed
-
- Averill S, Delcroix JD, Michael GJ, Tomlinson DR, Fernyhough P, Priestley JV. Nerve growth factor modulates the activation status and fast axonal transport of ERK 1/2 in adult nociceptive neurones. Mol Cell Neurosci. 2001;18:183–196. - PubMed
-
- Black JA, Dib-Hajj S, McNabola K, Jeste S, Rizzo MA, Kocsis JD, Waxman SG. Spinal sensory neurons express multiple sodium channel alpha-subunit mRNAs. Mol Brain Res. 1996;43:117–131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
