In the developing rat hippocampus, endogenous activation of presynaptic kainate receptors reduces GABA release from mossy fiber terminals
- PMID: 20130184
- PMCID: PMC6633972
- DOI: 10.1523/JNEUROSCI.4566-09.2010
In the developing rat hippocampus, endogenous activation of presynaptic kainate receptors reduces GABA release from mossy fiber terminals
Abstract
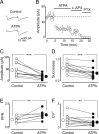
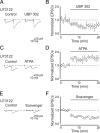
Presynaptic kainate receptors regulate synaptic transmission in several brain areas but are not known to have this action at immature mossy fiber (MF) terminals, which during the first week of postnatal life release GABA, which exerts into targeted cells a depolarizing and excitatory action. Here, we report that, during the first week of postnatal life, endogenous activation of GluK1 receptors by glutamate present in the extracellular space severely depresses MF-mediated GABAergic currents [GABA(A)-mediated postsynaptic currents (GPSCs)]. Activation of GluK1 receptors was prevented by treating the slices with enzymatic glutamate scavengers that enhanced the clearance of glutamate from the extracellular space. The depressant effect of GluK1 on MF-GPSCs was mediated by a metabotropic process sensitive to pertussis toxin. In the presence of U73122 (1-[6-[[(17b)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione), a selective inhibitor of phospholipase C, along the transduction pathway downstream to G-protein, GluK1 activation increased the probability of GABA release, thus unveiling the ionotropic action of this receptor. In line with this type of action, we found that GluK1 enhanced MF excitability by directly depolarizing MF terminals via calcium-permeable cation channels. Furthermore, GluK1 dynamically regulated the direction of spike time-dependent plasticity occurring by pairing MF stimulation with postsynaptic spiking and switched spike time-dependent potentiation into depression. The GluK1-induced depression of MF-GPSCs would prevent excessive activation of the CA3 associative network by the excitatory action of GABA and the emergence of seizures in the immature brain.
Figures






Similar articles
-
Control of GABA Release at Mossy Fiber-CA3 Connections in the Developing Hippocampus.Front Synaptic Neurosci. 2010 Feb 22;2:1. doi: 10.3389/neuro.19.001.2010. eCollection 2010. Front Synaptic Neurosci. 2010. PMID: 21423487 Free PMC article.
-
In the developing hippocampus kainate receptors control the release of GABA from mossy fiber terminals via a metabotropic type of action.Adv Exp Med Biol. 2011;717:11-26. doi: 10.1007/978-1-4419-9557-5_2. Adv Exp Med Biol. 2011. PMID: 21713663 Review.
-
At immature mossy-fiber-CA3 synapses, correlated presynaptic and postsynaptic activity persistently enhances GABA release and network excitability via BDNF and cAMP-dependent PKA.J Neurosci. 2009 Feb 25;29(8):2637-47. doi: 10.1523/JNEUROSCI.5019-08.2009. J Neurosci. 2009. PMID: 19244539 Free PMC article.
-
Target-cell specificity of kainate autoreceptor and Ca2+-store-dependent short-term plasticity at hippocampal mossy fiber synapses.J Neurosci. 2008 Dec 3;28(49):13139-49. doi: 10.1523/JNEUROSCI.2932-08.2008. J Neurosci. 2008. PMID: 19052205 Free PMC article.
-
Role of giant depolarizing potentials in shaping synaptic currents in the developing hippocampus.Crit Rev Neurobiol. 2006;18(1-2):13-23. doi: 10.1615/critrevneurobiol.v18.i1-2.30. Crit Rev Neurobiol. 2006. PMID: 17725505 Review.
Cited by
-
The Role of Phospholipase C in GABAergic Inhibition and Its Relevance to Epilepsy.Int J Mol Sci. 2021 Mar 19;22(6):3149. doi: 10.3390/ijms22063149. Int J Mol Sci. 2021. PMID: 33808762 Free PMC article. Review.
-
Electrophysiological characterization of granule cells in the dentate gyrus immediately after birth.Front Cell Neurosci. 2014 Feb 14;8:44. doi: 10.3389/fncel.2014.00044. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24592213 Free PMC article.
-
Control of GABA Release at Mossy Fiber-CA3 Connections in the Developing Hippocampus.Front Synaptic Neurosci. 2010 Feb 22;2:1. doi: 10.3389/neuro.19.001.2010. eCollection 2010. Front Synaptic Neurosci. 2010. PMID: 21423487 Free PMC article.
-
Mixed neurotransmission in the hippocampal mossy fibers.Front Cell Neurosci. 2013 Nov 22;7:210. doi: 10.3389/fncel.2013.00210. Front Cell Neurosci. 2013. PMID: 24319410 Free PMC article. Review.
-
Expression of GluK1c underlies the developmental switch in presynaptic kainate receptor function.Sci Rep. 2012;2:310. doi: 10.1038/srep00310. Epub 2012 Mar 12. Sci Rep. 2012. PMID: 22413061 Free PMC article.
References
-
- Amaral DG, Dent JA. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981;195:51–86. - PubMed
-
- Ben-Ari Y, Cossart R. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 2000;23:580–587. - PubMed
-
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
