The N-glycanase png-1 acts to limit axon branching during organ formation in Caenorhabditis elegans
- PMID: 20130186
- PMCID: PMC6634002
- DOI: 10.1523/JNEUROSCI.4962-08.2010
The N-glycanase png-1 acts to limit axon branching during organ formation in Caenorhabditis elegans
Abstract
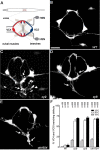
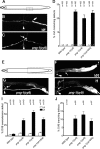
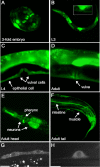
Peptide:N-glycanases (PNGases) are cytoplasmic de-N-glycosylation enzymes that have been shown in cultured cells to facilitate the degradation of misfolded glycoproteins during endoplasmic reticulum-associated degradation and in the processing of major histocompatibility complex class I antigens for proper cell-surface presentation. The gene encoding PNGase activity was initially described in budding yeast (Png1p) and shown to be highly conserved from yeast to humans, but physiological roles in higher organisms have not been elucidated. Here we describe peripheral nervous system defects associated with the first loss-of-function mutations in an animal PNGase. Mutations in png-1, the Caenorhabditis elegans PNGase ortholog, result in an increase in axon branching during morphogenesis of the vulval egg-laying organ and egg-laying behavior changes. Neuronal defects include an increase in the branched morphology of the VC4 and VC5 egg-laying neurons as well as inappropriate branches from axons that run adjacent to the vulva but would normally remain unbranched. We show that png-1 is widely expressed and can act from both neurons and epithelial cells to restrict axon branching. A deletion allele of the DNA repair gene rad-23, orthologs of which are known to physically interact with PNGases in yeast and mammals, displays similar axon branching defects and genetic interactions with png-1. In summary, our analysis reveals a novel developmental role for a PNGase and Rad-23 in the regulation of neuronal branching during organ innervation.
Figures






Similar articles
-
Dual enzymatic properties of the cytoplasmic peptide: N-glycanase in C. elegans.Biochem Biophys Res Commun. 2007 Jul 6;358(3):837-41. doi: 10.1016/j.bbrc.2007.04.199. Epub 2007 May 8. Biochem Biophys Res Commun. 2007. PMID: 17509531
-
Mutations in nucleotide metabolism genes bypass proteasome defects in png-1/NGLY1-deficient Caenorhabditis elegans.PLoS Biol. 2024 Jul 11;22(7):e3002720. doi: 10.1371/journal.pbio.3002720. eCollection 2024 Jul. PLoS Biol. 2024. PMID: 38991033 Free PMC article.
-
Unique peptide:N-glycanase of Caenorhabditis elegans has activity of protein disulphide reductase as well as of deglycosylation.J Biochem. 2007 Aug;142(2):175-81. doi: 10.1093/jb/mvm117. Epub 2007 May 23. J Biochem. 2007. PMID: 17522090
-
The cytoplasmic peptide:N-glycanase (Ngly1)-basic science encounters a human genetic disorder.J Biochem. 2015 Jan;157(1):23-34. doi: 10.1093/jb/mvu068. Epub 2014 Nov 13. J Biochem. 2015. PMID: 25398991 Review.
-
The cytoplasmic peptide:N-glycanase (NGLY1) - Structure, expression and cellular functions.Gene. 2016 Feb 10;577(1):1-7. doi: 10.1016/j.gene.2015.11.021. Epub 2015 Nov 30. Gene. 2016. PMID: 26611529 Free PMC article. Review.
Cited by
-
Interplay between redox and protein homeostasis.Worm. 2016 Mar 30;5(2):e1170273. doi: 10.1080/21624054.2016.1170273. eCollection 2016 Apr-Jun. Worm. 2016. PMID: 27386166 Free PMC article. Review.
-
The glutathione system and the related thiol network in Caenorhabditis elegans.Redox Biol. 2019 Jun;24:101171. doi: 10.1016/j.redox.2019.101171. Epub 2019 Mar 16. Redox Biol. 2019. PMID: 30901603 Free PMC article. Review.
-
Ngly1 -/- rats develop neurodegenerative phenotypes and pathological abnormalities in their peripheral and central nervous systems.Hum Mol Genet. 2020 Jun 27;29(10):1635-1647. doi: 10.1093/hmg/ddaa059. Hum Mol Genet. 2020. PMID: 32259258 Free PMC article.
-
JF1/B6F1 Ngly1-/- mouse as an isogenic animal model of NGLY1 deficiency.Proc Jpn Acad Ser B Phys Biol Sci. 2021;97(2):89-102. doi: 10.2183/pjab.97.005. Proc Jpn Acad Ser B Phys Biol Sci. 2021. PMID: 33563880 Free PMC article.
-
Functional characterization of thioredoxin 3 (TRX-3), a Caenorhabditis elegans intestine-specific thioredoxin.Free Radic Biol Med. 2014 Mar;68(100):205-19. doi: 10.1016/j.freeradbiomed.2013.11.023. Epub 2013 Dec 4. Free Radic Biol Med. 2014. PMID: 24316195 Free PMC article.
References
-
- Altrich-VanLith ML, Ostankovitch M, Polefrone JM, Mosse CA, Shabanowitz J, Hunt DF, Engelhard VH. Processing of a class I-restricted epitope from tyrosinase requires peptide N-glycanase and the cooperative action of endoplasmic reticulum aminopeptidase 1 and cytosolic proteases. J Immunol. 2006;177:5440–5450. - PubMed
-
- Altun-Gultekin Z, Andachi Y, Tsalik EL, Pilgrim D, Kohara Y, Hobert O. A regulatory cascade of three homeobox genes, ceh-10, ttx-3 and ceh-23, controls cell fate specification of a defined interneuron class in C. elegans. Development. 2001;128:1951–1969. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
