Presynaptic mitochondria in functionally different motor neurons exhibit similar affinities for Ca2+ but exert little influence as Ca2+ buffers at nerve firing rates in situ
- PMID: 20130196
- PMCID: PMC2824909
- DOI: 10.1523/JNEUROSCI.4701-09.2010
Presynaptic mitochondria in functionally different motor neurons exhibit similar affinities for Ca2+ but exert little influence as Ca2+ buffers at nerve firing rates in situ
Abstract
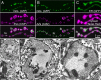
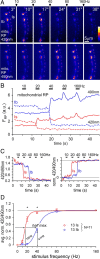
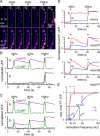
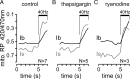
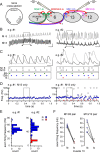
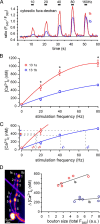
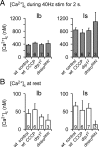
Mitochondria accumulate within nerve terminals and support synaptic function, most notably through ATP production. They can also sequester Ca(2+) during nerve stimulation, but it is unknown whether this limits presynaptic Ca(2+) levels at physiological nerve firing rates. Similarly, it is unclear whether mitochondrial Ca(2+) sequestration differs between functionally different nerve terminals. We addressed these questions using a combination of synthetic and genetically encoded Ca(2+) indicators to examine cytosolic and mitochondrial Ca(2+) levels in presynaptic terminals of tonic (MN13-Ib) and phasic (MNSNb/d-Is) motor neurons in Drosophila, which, as we determined, fire during fictive locomotion at approximately 42 Hz and approximately 8 Hz, respectively. Mitochondrial Ca(2+) sequestration starts in both terminals at approximately 250 nM, exhibits a similar Ca(2+)-uptake affinity (approximately 410 nM), and does not require Ca(2+) release from the endoplasmic reticulum. Nonetheless, mitochondrial Ca(2+) uptake in type Is terminals is more responsive to low-frequency nerve stimulation and this is due to higher cytosolic Ca(2+) levels. Since type Ib terminals have a higher mitochondrial density than Is terminals, it seemed possible that greater mitochondrial Ca(2+) sequestration may be responsible for the lower cytosolic Ca(2+) levels in Ib terminals. However, genetic and pharmacological manipulations of mitochondrial Ca(2+) uptake did not significantly alter nerve-stimulated elevations in cytosolic Ca(2+) levels in either terminal type within physiologically relevant rates of stimulation. Our findings indicate that presynaptic mitochondria have a similar affinity for Ca(2+) in functionally different nerve terminals, but do not limit cytosolic Ca(2+) levels within the range of motor neuron firing rates in situ.
Figures

Similar articles
-
Mitochondrial Ca2+ uptake prevents desynchronization of quantal release and minimizes depletion during repetitive stimulation of mouse motor nerve terminals.J Physiol. 2003 Apr 15;548(Pt 2):425-38. doi: 10.1113/jphysiol.2002.035196. Epub 2003 Feb 14. J Physiol. 2003. PMID: 12588898 Free PMC article.
-
Cytosolic calcium coordinates mitochondrial energy metabolism with presynaptic activity.J Neurosci. 2012 Jan 25;32(4):1233-43. doi: 10.1523/JNEUROSCI.1301-11.2012. J Neurosci. 2012. PMID: 22279208 Free PMC article.
-
Inhibition of mitochondrial Ca2+ uptake affects phasic release from motor terminals differently depending on external [Ca2+].J Neurophysiol. 2003 Jul;90(1):491-502. doi: 10.1152/jn.00012.2003. Epub 2003 Apr 2. J Neurophysiol. 2003. PMID: 12672777
-
Presynaptic excitability.Int Rev Neurobiol. 1995;38:201-51. doi: 10.1016/s0074-7742(08)60527-9. Int Rev Neurobiol. 1995. PMID: 8537201 Review.
-
Mitochondria in motor nerve terminals: function in health and in mutant superoxide dismutase 1 mouse models of familial ALS.J Bioenerg Biomembr. 2011 Dec;43(6):581-6. doi: 10.1007/s10863-011-9392-1. J Bioenerg Biomembr. 2011. PMID: 22089637 Free PMC article. Review.
Cited by
-
Proportional Downscaling of Glutamatergic Release Sites by the General Anesthetic Propofol at Drosophila Motor Nerve Terminals.eNeuro. 2020 Feb 28;7(1):ENEURO.0422-19.2020. doi: 10.1523/ENEURO.0422-19.2020. Print 2020 Jan/Feb. eNeuro. 2020. PMID: 32019872 Free PMC article.
-
The role of oxidative stress in degeneration of the neuromuscular junction in amyotrophic lateral sclerosis.Front Cell Neurosci. 2014 May 13;8:131. doi: 10.3389/fncel.2014.00131. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24860432 Free PMC article. Review.
-
Physiologic and Nanoscale Distinctions Define Glutamatergic Synapses in Tonic vs Phasic Neurons.J Neurosci. 2023 Jun 21;43(25):4598-4611. doi: 10.1523/JNEUROSCI.0046-23.2023. Epub 2023 May 23. J Neurosci. 2023. PMID: 37221096 Free PMC article.
-
Transmission, Development, and Plasticity of Synapses.Genetics. 2015 Oct;201(2):345-75. doi: 10.1534/genetics.115.176529. Genetics. 2015. PMID: 26447126 Free PMC article. Review.
-
EndophilinA-dependent coupling between activity-induced calcium influx and synaptic autophagy is disrupted by a Parkinson-risk mutation.Neuron. 2023 May 3;111(9):1402-1422.e13. doi: 10.1016/j.neuron.2023.02.001. Epub 2023 Feb 23. Neuron. 2023. PMID: 36827984 Free PMC article.
References
-
- Abad MF, Di Benedetto G, Magalhães PJ, Filippin L, Pozzan T. Mitochondrial pH monitored by a new engineered green fluorescent protein mutant. J Biol Chem. 2004;279:11521–11529. - PubMed
-
- Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhães TR, Goodman CS. wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron. 2002;33:545–558. - PubMed
-
- Alonso MT, Villalobos C, Chamero P, Alvarez J, García-Sancho J. Calcium microdomains in mitochondria and nucleus. Cell Calcium. 2006;40:513–525. - PubMed
-
- Atwood HL, Govind CK, Wu CF. Differential ultrastructure of synaptic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. J Neurobiol. 1993;24:1008–1024. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
