Vibrissa-based object localization in head-fixed mice
- PMID: 20130203
- PMCID: PMC6634009
- DOI: 10.1523/JNEUROSCI.3762-09.2010
Vibrissa-based object localization in head-fixed mice
Abstract
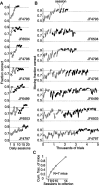
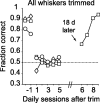
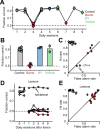
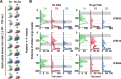
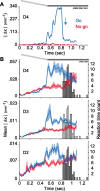
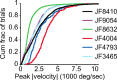
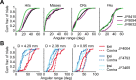
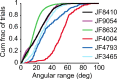
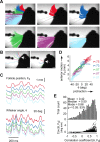
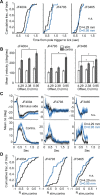
Linking activity in specific cell types with perception, cognition, and action, requires quantitative behavioral experiments in genetic model systems such as the mouse. In head-fixed primates, the combination of precise stimulus control, monitoring of motor output, and physiological recordings over large numbers of trials are the foundation on which many conceptually rich and quantitative studies have been built. Choice-based, quantitative behavioral paradigms for head-fixed mice have not been described previously. Here, we report a somatosensory absolute object localization task for head-fixed mice. Mice actively used their mystacial vibrissae (whiskers) to sense the location of a vertical pole presented to one side of the head and reported with licking whether the pole was in a target (go) or a distracter (no-go) location. Mice performed hundreds of trials with high performance (>90% correct) and localized to <0.95 mm (<6 degrees of azimuthal angle). Learning occurred over 1-2 weeks and was observed both within and across sessions. Mice could perform object localization with single whiskers. Silencing barrel cortex abolished performance to chance levels. We measured whisker movement and shape for thousands of trials. Mice moved their whiskers in a highly directed, asymmetric manner, focusing on the target location. Translation of the base of the whiskers along the face contributed substantially to whisker movements. Mice tended to maximize contact with the go (rewarded) stimulus while minimizing contact with the no-go stimulus. We conjecture that this may amplify differences in evoked neural activity between trial types.
Figures










References
-
- Berg RW, Kleinfeld D. Rhythmic whisking by rat: retraction as well as protraction of the vibrissae is under active muscular control. J Neurophysiol. 2003;89:104–117. - PubMed
-
- Birdwell JA, Solomon JH, Thajchayapong M, Taylor MA, Cheely M, Towal RB, Conradt J, Hartmann MJ. Biomechanical models for radial distance determination by the rat vibrissal system. J Neurophysiol. 2007;98:2439–2455. - PubMed
-
- Boyden ES, Raymond JL. Active reversal of motor memories reveals rules governing memory encoding. Neuron. 2003;39:1031–1042. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
