HCO (3)(-) secretion and CaCO3 precipitation play major roles in intestinal water absorption in marine teleost fish in vivo
- PMID: 20130226
- PMCID: PMC2853399
- DOI: 10.1152/ajpregu.00545.2009
HCO (3)(-) secretion and CaCO3 precipitation play major roles in intestinal water absorption in marine teleost fish in vivo
Abstract
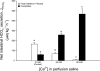
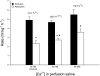
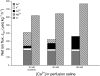
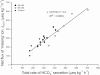
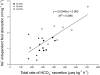
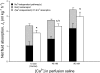
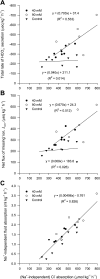
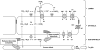
The intestine of marine teleosts must effectively absorb fluid from ingested seawater to avoid dehydration. This fluid transport has been almost exclusively characterized as driven by NaCl absorption. However, an additional feature of the osmoregulatory role of the intestine is substantial net HCO(3)(-) secretion. This is suggested to drive additional fluid absorption directly (via Cl(-)/HCO(3)(-) exchange) and indirectly by precipitating ingested Ca(2+) as CaCO(3), thus creating the osmotic gradient for additional fluid absorption. The present study tested this hypothesis by perfusing the intestine of the European flounder in vivo with varying [Ca(2+)]: 10 (control), 40, and 90 mM. Fractional fluid absorption increased from 47% (control) to 73% (90 mM Ca(2+)), where almost all secreted HCO(3)(-) was excreted as CaCO(3). This additional fluid absorption could not be explained by NaCl cotransport. Instead, a significant positive relationship between Na(+)-independent fluid absorption and total HCO(3)(-) secretion was consistent with the predicted roles for anion exchange and CaCO(3) precipitation. Further analysis suggested that Na(+)-independent fluid absorption could be accounted for by net Cl(-) and H(+) absorption (from Cl(-)/HCO(3)(-) exchange and CO(2) hydration, respectively). There was no evidence to suggest that CaCO(3) alone was responsible for driving fluid absorption. However, by preventing the accumulation of luminal Ca(2+) it played a vital role by dynamically maintaining a favorable osmotic gradient all along the intestine, which permits substantially higher rates of solute-linked fluid absorption. To overcome the resulting hyperosmotic and highly acidic absorbate, it is proposed that plasma HCO(3)(-) buffers the absorbed H(+) (from HCO(3)(-) production), and consequently reduces the osmolarity of the absorbed fluid entering the body.
Figures
Similar articles
-
Ca2+-driven intestinal HCO(3)(-) secretion and CaCO3 precipitation in the European flounder in vivo: influences on acid-base regulation and blood gas transport.Am J Physiol Regul Integr Comp Physiol. 2010 Apr;298(4):R870-6. doi: 10.1152/ajpregu.00513.2009. Epub 2010 Feb 3. Am J Physiol Regul Integr Comp Physiol. 2010. PMID: 20130227 Free PMC article.
-
Intestinal bicarbonate secretion in marine teleost fish-source of bicarbonate, pH sensitivity, and consequences for whole animal acid-base and calcium homeostasis.Biochim Biophys Acta. 2003 Dec 30;1618(2):163-74. doi: 10.1016/j.bbamem.2003.09.014. Biochim Biophys Acta. 2003. PMID: 14729153
-
Intestinal bicarbonate secretion by marine teleost fish--why and how?Biochim Biophys Acta. 2002 Nov 13;1566(1-2):182-93. doi: 10.1016/s0005-2736(02)00600-4. Biochim Biophys Acta. 2002. PMID: 12421549 Review.
-
The influence of 17β-estradiol on intestinal calcium carbonate precipitation and osmoregulation in seawater-acclimated rainbow trout (Oncorhynchus mykiss).J Exp Biol. 2011 Aug 15;214(Pt 16):2791-8. doi: 10.1242/jeb.054296. J Exp Biol. 2011. PMID: 21795578
-
Intestinal anion exchange in marine teleosts is involved in osmoregulation and contributes to the oceanic inorganic carbon cycle.Acta Physiol (Oxf). 2011 Jul;202(3):421-34. doi: 10.1111/j.1748-1716.2010.02241.x. Epub 2011 Mar 1. Acta Physiol (Oxf). 2011. PMID: 21362153 Review.
Cited by
-
Metabolic responses of the Antarctic fishes Notothenia rossii and Notothenia coriiceps to sewage pollution.Fish Physiol Biochem. 2015 Oct;41(5):1205-20. doi: 10.1007/s10695-015-0080-7. Epub 2015 Jun 2. Fish Physiol Biochem. 2015. PMID: 26031510
-
Osmoregulation and epithelial water transport: lessons from the intestine of marine teleost fish.J Comp Physiol B. 2012 Jan;182(1):1-39. doi: 10.1007/s00360-011-0601-3. Epub 2011 Jul 7. J Comp Physiol B. 2012. PMID: 21735220 Review.
-
Calcium-sensing receptor: A new target for therapy of diarrhea.World J Gastroenterol. 2016 Mar 7;22(9):2711-24. doi: 10.3748/wjg.v22.i9.2711. World J Gastroenterol. 2016. PMID: 26973410 Free PMC article. Review.
-
Biogenic fish-gut calcium carbonate is a stable amorphous phase in the gilt-head seabream, Sparus aurata.Sci Rep. 2013;3:1700. doi: 10.1038/srep01700. Sci Rep. 2013. PMID: 23609008 Free PMC article.
-
Di- and tripeptide transport in vertebrates: the contribution of teleost fish models.J Comp Physiol B. 2017 Apr;187(3):395-462. doi: 10.1007/s00360-016-1044-7. Epub 2016 Nov 1. J Comp Physiol B. 2017. PMID: 27803975 Review.
References
-
- Altimiras J, Claireaux G, Sandblom E, Farrell AP, McKenzie DJ, Axelsson M. Gastrointestinal blood flow and postprandial metabolism in swimming sea bassDicentrarchus labrax. Physiol Biochem Zool 81: 663–672, 2008 - PubMed
-
- Ando M, Subramanyam MVV. Bicarbonate transport systems in the intestine of the seawater eel. J Exp Biol 150: 381–394, 1990
-
- Axelsson M, Thorarensen H, Nilsson S, Farrell AP. Gastrointestinal blood flow in the red Irish lord, Hemilepidotus hemilepidotus: long-term effects of feeding and adrenergic control. J Comp Physiol B 170: 145–152, 2000 - PubMed
-
- Bucking C, Fitzpatrick JL, Nadella SR, Wood CM. Post-prandial metabolic alkalosis in the seawater-acclimated trout: the alkaline tide comes in. J Exp Biol 212: 2159–2166, 2009 - PubMed
-
- Bucking C, Wood CM. The alkaline tide and ammonia excretion after voluntary feeding in freshwater rainbow trout. J Exp Biol 211: 2533–2541, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

