Impaired NK-cell migration in WAS/XLT patients: role of Cdc42/WASp pathway in the control of chemokine-induced beta2 integrin high-affinity state
- PMID: 20130240
- PMCID: PMC2854428
- DOI: 10.1182/blood-2009-07-235804
Impaired NK-cell migration in WAS/XLT patients: role of Cdc42/WASp pathway in the control of chemokine-induced beta2 integrin high-affinity state
Abstract
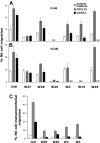
We analyzed the involvement of Wiskott-Aldrich syndrome protein (WASp), a critical regulator of actin cytoskeleton remodeling, in the control of natural killer (NK)-cell migration. NK cells derived from patients with Wiskott-Aldrich syndrome/X-linked thrombocytopenia (WAS/XLT), carrying different mutations in the WASP coding gene, displayed reduced migration through intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), or endothelial cells in response to CXCL12/stromal cell-derived factor-1 and CX3CL1/fractalkine. Inhibition of WAS/XLT NK-cell migration was associated with reduced ability of these cells to up-regulate the expression of CD18 activation neoepitope and to adhere to ICAM-1 or VCAM-1 following chemokine stimulation. Moreover, chemokine receptor or beta1 or beta2 integrin engagement on NK cells rapidly resulted in Cdc42 activation and WASp tyrosine phosphorylation as well as in WASp association with Fyn and Pyk-2 tyrosine kinases. NK-cell pretreatment with wiskostatin, to prevent Cdc42/WASp association, impaired chemokine-induced NK-cell migration through ICAM-1 and beta2 integrin activation-dependent neoepitope expression. These results show that the Cdc42/WASp pathway plays a crucial role in the regulation of NK-cell migration by acting as a critical component of the chemokine-induced inside-out signaling that regulates lymphocyte function-associated antigen-1 function and suggest that after integrin or chemokine receptor engagement WASp function is regulated by the coordinate action of both Cdc42 and tyrosine kinases.
Figures
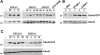

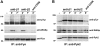
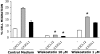

Similar articles
-
Impaired natural and CD16-mediated NK cell cytotoxicity in patients with WAS and XLT: ability of IL-2 to correct NK cell functional defect.Blood. 2004 Jul 15;104(2):436-43. doi: 10.1182/blood-2003-07-2621. Epub 2004 Mar 4. Blood. 2004. PMID: 15001467
-
Mutations of the Wiskott-Aldrich Syndrome Protein affect protein expression and dictate the clinical phenotypes.Immunol Res. 2009;44(1-3):84-8. doi: 10.1007/s12026-008-8084-3. Immunol Res. 2009. PMID: 19082760 Review.
-
WIP remodeling actin behind the scenes: how WIP reshapes immune and other functions.Int J Mol Sci. 2012;13(6):7629-7647. doi: 10.3390/ijms13067629. Epub 2012 Jun 21. Int J Mol Sci. 2012. PMID: 22837718 Free PMC article. Review.
-
The Wiskott-Aldrich syndrome: The actin cytoskeleton and immune cell function.Dis Markers. 2010;29(3-4):157-75. doi: 10.3233/DMA-2010-0735. Dis Markers. 2010. PMID: 21178275 Free PMC article. Review.
-
Proline-rich tyrosine kinase 2 and Rac activation by chemokine and integrin receptors controls NK cell transendothelial migration.J Immunol. 2003 Mar 15;170(6):3065-73. doi: 10.4049/jimmunol.170.6.3065. J Immunol. 2003. PMID: 12626562
Cited by
-
Tyrosine phosphorylation of Wiskott-Aldrich syndrome protein (WASP) by Hck regulates macrophage function.J Biol Chem. 2014 Mar 14;289(11):7897-906. doi: 10.1074/jbc.M113.509497. Epub 2014 Jan 30. J Biol Chem. 2014. PMID: 24482227 Free PMC article.
-
Role of WASP in cell polarity and podosome dynamics of myeloid cells.Eur J Cell Biol. 2011 Feb-Mar;90(2-3):198-204. doi: 10.1016/j.ejcb.2010.05.009. Epub 2010 Jul 6. Eur J Cell Biol. 2011. PMID: 20609498 Free PMC article. Review.
-
Targeting the actin nucleation promoting factor WASp provides a therapeutic approach for hematopoietic malignancies.Nat Commun. 2021 Sep 22;12(1):5581. doi: 10.1038/s41467-021-25842-7. Nat Commun. 2021. PMID: 34552085 Free PMC article.
-
Wiskott-Aldrich syndrome protein controls antigen-presenting cell-driven CD4+ T-cell motility by regulating adhesion to intercellular adhesion molecule-1.Immunology. 2012 Oct;137(2):183-96. doi: 10.1111/j.1365-2567.2012.03620.x. Immunology. 2012. PMID: 22804504 Free PMC article.
-
Activation of Lymphocyte Cytolytic Machinery: Where are We?Front Immunol. 2013 Nov 19;4:390. doi: 10.3389/fimmu.2013.00390. Front Immunol. 2013. PMID: 24312097 Free PMC article. Review.
References
-
- Thrasher AJ. WASp in immune-system organization and function. Nat Rev Immunol. 2002;2(9):635–646. - PubMed
-
- Badour K, Zhang J, Siminovitch KA. The Wiskott-Aldrich syndrome protein: forging the link between actin and cell activation. Immunol Rev. 2003;192:98–112. - PubMed
-
- Villa A, Notarangelo L, Macchi P, et al. X-linked thrombocytopenia and Wiskott-Aldrich syndrome are allelic diseases with mutations in the WASP gene. Nat Genet. 1995;9(4):414–417. - PubMed
-
- Zhu Q, Watanabe C, Liu T, et al. Wiskott-Aldrich syndrome/X-linked thrombocytopenia: WASP gene mutations, protein expression and phenotype. Blood. 1997;90(7):2680–2689. - PubMed
-
- Ochs HD, Rosen FS. The Wiskott-Aldrich syndrome. In: Ochs HD, Smith CIE, Puck JM, editors. Primary Immunodeficiency Diseases: A Molecular and Genetics Approach. New York, NY: Oxford University Press; 1999. pp. 292–305.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

