Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms
- PMID: 20130243
- PMCID: PMC3953826
- DOI: 10.1182/blood-2009-04-214957
Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms
Abstract
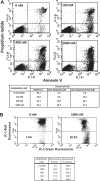
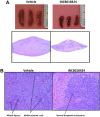
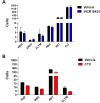
Constitutive JAK2 activation in hematopoietic cells by the JAK2V617F mutation recapitulates myeloproliferative neoplasm (MPN) phenotypes in mice, establishing JAK2 inhibition as a potential therapeutic strategy. Although most polycythemia vera patients carry the JAK2V617F mutation, half of those with essential thrombocythemia or primary myelofibrosis do not, suggesting alternative mechanisms for constitutive JAK-STAT signaling in MPNs. Most patients with primary myelofibrosis have elevated levels of JAK-dependent proinflammatory cytokines (eg, interleukin-6) consistent with our observation of JAK1 hyperactivation. Accordingly, we evaluated the effectiveness of selective JAK1/2 inhibition in experimental models relevant to MPNs and report on the effects of INCB018424, the first potent, selective, oral JAK1/JAK2 inhibitor to enter the clinic. INCB018424 inhibited interleukin-6 signaling (50% inhibitory concentration [IC(50)] = 281nM), and proliferation of JAK2V617F(+) Ba/F3 cells (IC(50) = 127nM). In primary cultures, INCB018424 preferentially suppressed erythroid progenitor colony formation from JAK2V617F(+) polycythemia vera patients (IC(50) = 67nM) versus healthy donors (IC(50) > 400nM). In a mouse model of JAK2V617F(+) MPN, oral INCB018424 markedly reduced splenomegaly and circulating levels of inflammatory cytokines, and preferentially eliminated neoplastic cells, resulting in significantly prolonged survival without myelosuppressive or immunosuppressive effects. Preliminary clinical results support these preclinical data and establish INCB018424 as a promising oral agent for the treatment of MPNs.
Figures


References
-
- Prchal JF, Axelrad AA. Bone-marrow responses in polycythemia vera [Letter]. N Engl J Med. 1974;290(24):1382. - PubMed
-
- Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–1061. - PubMed
-
- James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–1148. - PubMed
-
- Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–1790. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

