Growth factor signaling in vitreous humor-induced lens fiber differentiation
- PMID: 20130274
- PMCID: PMC2904012
- DOI: 10.1167/iovs.09-4797
Growth factor signaling in vitreous humor-induced lens fiber differentiation
Abstract
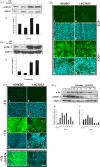
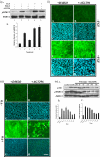
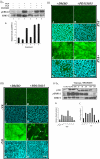
PURPOSE. Although some of the factors and signaling pathways that are involved in induction of fiber differentiation have been defined, such as FGF-mediated MAPK/ERK and PI3-K/Akt signaling, the factors in the vitreous that regulate this differentiation process in vivo have yet to be identified. The purpose of this study was to better understand the role of growth factors in vitreous that regulate this process by further characterizing the signaling pathways involved in lens fiber differentiation. METHODS. Rat lens epithelial explants were used to compare the ability of vitreous, IGF-1, PDGF-A, EGF, and FGF-2 to stimulate the phosphorylation of ERK1/2 and Akt leading to fiber differentiation, in the presence or absence of selective receptor tyrosine kinase (RTK) inhibitors. RESULTS. Similar to vitreous, FGF induced a sustained ERK1/2 signaling profile, unlike IGF, PDGF, and EGF, which induced a more transient (shorter) activation of ERK1/2. For Akt activation, IGF was the only factor that induced a profile similar to vitreous. IGF, PDGF, and EGF potentiated the effects of a low dose of FGF on lens fiber differentiation by extending the duration of ERK1/2 phosphorylation. In the presence of selective RTK inhibitors, although the sustained vitreous-induced ERK1/2 signaling profile and subsequent fiber differentiation was perturbed, the results also showed that, although prolonged ERK1/2 phosphorylation was necessary, it was not sufficient for fiber differentiation to proceed. CONCLUSIONS. These results are consistent with FGF's being the key growth factor involved in vitreous-induced signaling leading to lens fiber differentiation; however, they also indicate that other vitreal growth factors such as IGF may be involved in fine-tuning ERK1/2- and Akt-phosphorylation to the level that is necessary for initiation and/or maintenance of lens fiber differentiation in vivo.
Figures

Similar articles
-
Evaluation of fibroblast growth factor signaling during lens fiber cell differentiation.Invest Ophthalmol Vis Sci. 2003 Feb;44(2):680-90. doi: 10.1167/iovs.01-1177. Invest Ophthalmol Vis Sci. 2003. PMID: 12556399
-
Duration of ERK1/2 phosphorylation induced by FGF or ocular media determines lens cell fate.Differentiation. 2007 Sep;75(7):662-8. doi: 10.1111/j.1432-0436.2007.00167.x. Epub 2007 Mar 23. Differentiation. 2007. PMID: 17381542
-
Growth factors involved in aqueous humour-induced lens cell proliferation.Growth Factors. 2009 Feb;27(1):50-62. doi: 10.1080/08977190802610916. Growth Factors. 2009. PMID: 19085197
-
Fibrosis in the lens. Sprouty regulation of TGFβ-signaling prevents lens EMT leading to cataract.Exp Eye Res. 2016 Jan;142:92-101. doi: 10.1016/j.exer.2015.02.004. Epub 2015 May 21. Exp Eye Res. 2016. PMID: 26003864 Free PMC article. Review.
-
The role of fibroblast growth factor in eye lens development.Ann N Y Acad Sci. 1991;638:256-74. doi: 10.1111/j.1749-6632.1991.tb49036.x. Ann N Y Acad Sci. 1991. PMID: 1723855 Review.
Cited by
-
Tonic ErbB signaling underlies TGFβ-induced activation of ERK and is required for lens cell epithelial to myofibroblast transition.Mol Biol Cell. 2024 Mar 1;35(3):ar35. doi: 10.1091/mbc.E23-07-0294. Epub 2024 Jan 3. Mol Biol Cell. 2024. PMID: 38170570 Free PMC article.
-
Loss of Dlg-1 in the mouse lens impairs fibroblast growth factor receptor signaling.PLoS One. 2014 May 13;9(5):e97470. doi: 10.1371/journal.pone.0097470. eCollection 2014. PLoS One. 2014. PMID: 24824078 Free PMC article.
-
Comparison of laser and ozone treatments on oral mucositis in an experimental model.Lasers Med Sci. 2017 Apr;32(3):673-677. doi: 10.1007/s10103-017-2166-1. Epub 2017 Feb 11. Lasers Med Sci. 2017. PMID: 28190112
-
Prox1 and fibroblast growth factor receptors form a novel regulatory loop controlling lens fiber differentiation and gene expression.Development. 2016 Jan 15;143(2):318-28. doi: 10.1242/dev.127860. Epub 2015 Dec 10. Development. 2016. PMID: 26657765 Free PMC article.
-
Differential regulation of Connexin50 and Connexin46 by PI3K signaling.FEBS Lett. 2015 May 22;589(12):1340-5. doi: 10.1016/j.febslet.2015.04.029. Epub 2015 Apr 29. FEBS Lett. 2015. PMID: 25935417 Free PMC article.
References
-
- Coulombre JL, Coulombre AJ. Lens development: fiber elongation and lens orientation. Science 1963;142:1489–1490 - PubMed
-
- McAvoy JW, Chamberlain CG. Growth factors in the eye. Prog Growth Factor Res 1990;2:29–43 - PubMed
-
- Schulz MW, Chamberlain CG, de Iongh RU, McAvoy JW. Acidic and basic FGF in ocular media and lens: implications for lens polarity and growth patterns. Development 1993;118:117–126 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

