Tamm-Horsfall protein and urinary exosome isolation
- PMID: 20130532
- PMCID: PMC3398710
- DOI: 10.1038/ki.2009.550
Tamm-Horsfall protein and urinary exosome isolation
Abstract
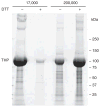
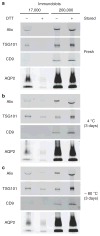
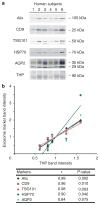
Urinary exosomes have been proposed as starting material for discovery of protein biomarkers of kidney disease. Current protocols for their isolation use a two-step differential centrifugation process. Due to their low density, exosomes are expected to remain in the low-speed (17,000 x g) supernatant and to sediment only when the sample is spun at high speed (200,000 x g). Analysis using western blot and electron microscopy found that urinary exosomes are also present in the low-speed pellet entrapped by polymeric Tamm-Horsfall protein, thus diminishing the procedure's reproducibility. Here we show that addition of dithiothreitol to the low-speed pellet disrupted the polymeric network, presumably by reduction of disulfide bonds linking the monomers. This modification shifted the exosomal proteins from the low- to the high-speed pellet. Also, by shifting the Tamm-Horsfall protein to the high-speed pellet, the use of dithiothreitol makes it feasible to use Tamm-Horsfall protein to normalize excretion rates of exosomal proteins in spot urines. We tested this by western blot, and found that there was a high degree of correlation between exosomal proteins and Tamm-Horsfall protein in the high-speed pellet. Since the yield of exosomes by differential centrifugation can be increased by chemical reduction, Tamm-Horsfall protein may be a suitable normalizing variable for urinary exosome studies when quantitative urine collections are not practical.
Conflict of interest statement
All the authors declared no competing interests.
Figures




References
-
- Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008;8:4083–4099. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

