CHD7 cooperates with PBAF to control multipotent neural crest formation
- PMID: 20130577
- PMCID: PMC2890258
- DOI: 10.1038/nature08733
CHD7 cooperates with PBAF to control multipotent neural crest formation
Abstract
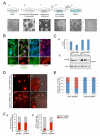
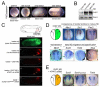
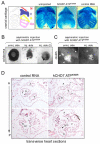
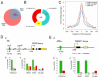
Heterozygous mutations in the gene encoding the CHD (chromodomain helicase DNA-binding domain) member CHD7, an ATP-dependent chromatin remodeller homologous to the Drosophila trithorax-group protein Kismet, result in a complex constellation of congenital anomalies called CHARGE syndrome, which is a sporadic, autosomal dominant disorder characterized by malformations of the craniofacial structures, peripheral nervous system, ears, eyes and heart. Although it was postulated 25 years ago that CHARGE syndrome results from the abnormal development of the neural crest, this hypothesis remained untested. Here we show that, in both humans and Xenopus, CHD7 is essential for the formation of multipotent migratory neural crest (NC), a transient cell population that is ectodermal in origin but undergoes a major transcriptional reprogramming event to acquire a remarkably broad differentiation potential and ability to migrate throughout the body, giving rise to craniofacial bones and cartilages, the peripheral nervous system, pigmentation and cardiac structures. We demonstrate that CHD7 is essential for activation of the NC transcriptional circuitry, including Sox9, Twist and Slug. In Xenopus embryos, knockdown of Chd7 or overexpression of its catalytically inactive form recapitulates all major features of CHARGE syndrome. In human NC cells CHD7 associates with PBAF (polybromo- and BRG1-associated factor-containing complex) and both remodellers occupy a NC-specific distal SOX9 enhancer and a conserved genomic element located upstream of the TWIST1 gene. Consistently, during embryogenesis CHD7 and PBAF cooperate to promote NC gene expression and cell migration. Our work identifies an evolutionarily conserved role for CHD7 in orchestrating NC gene expression programs, provides insights into the synergistic control of distal elements by chromatin remodellers, illuminates the patho-embryology of CHARGE syndrome, and suggests a broader function for CHD7 in the regulation of cell motility.
Figures


References
-
- Mohn F, Schubeler D. Genetics and epigenetics: stability and plasticity during cellular differentiation. Trends Genet. 2009;25:129–136. - PubMed
-
- Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008;9:557–568. - PubMed
-
- Dupin E, Creuzet S, Le Douarin NM. The contribution of the neural crest to the vertebrate body. Adv Exp Med Biol. 2006;589:96–119. - PubMed
-
- Surani MA, Hayashi K, Hajkova P. Genetic and epigenetic regulators of pluripotency. Cell. 2007;128:747–762. - PubMed
Methods references
-
- Lee G, et al. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol. 2007;25:1468–1475. - PubMed
-
- Zufferey R, Nagy D, Mandel R, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–875. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

