Plasmepsin V licenses Plasmodium proteins for export into the host erythrocyte
- PMID: 20130644
- PMCID: PMC2826791
- DOI: 10.1038/nature08726
Plasmepsin V licenses Plasmodium proteins for export into the host erythrocyte
Abstract
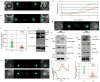
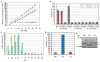
During their intraerythrocytic development, malaria parasites export hundreds of proteins to remodel their host cell. Nutrient acquisition, cytoadherence and antigenic variation are among the key virulence functions effected by this erythrocyte takeover. Proteins destined for export are synthesized in the endoplasmic reticulum (ER) and cleaved at a conserved (PEXEL) motif, which allows translocation into the host cell via an ATP-driven translocon called the PTEX complex. We report that plasmepsin V, an ER aspartic protease with distant homology to the mammalian processing enzyme BACE, recognizes the PEXEL motif and cleaves it at the correct site. This enzyme is essential for parasite viability and ER residence is essential for its function. We propose that plasmepsin V is the PEXEL protease and is an attractive enzyme for antimalarial drug development.
Conflict of interest statement
Competing financial interests: none.
Figures




References
-
- Haldar K, Mohandas N. Erythrocyte remodeling by malaria parasites. Current Opinion Hematol. 2007;14:203–209. - PubMed
-
- Maier AG, Cooke BM, Cowman AF, Tilley L. Malaria parasite proteins that remodel the host erythrocyte. Nature Reviews. 2009;7:341–354. - PubMed
-
- Hiller NL, et al. A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science. 2004;306:1934–1937. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

