Early Visually Evoked Electrophysiological Responses Over the Human Brain (P1, N170) Show Stable Patterns of Face-Sensitivity from 4 years to Adulthood
- PMID: 20130759
- PMCID: PMC2805434
- DOI: 10.3389/neuro.09.067.2009
Early Visually Evoked Electrophysiological Responses Over the Human Brain (P1, N170) Show Stable Patterns of Face-Sensitivity from 4 years to Adulthood
Abstract
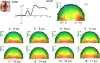
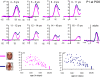
Whether the development of face recognition abilities truly reflects changes in how faces, specifically, are perceived, or rather can be attributed to more general perceptual or cognitive development, is debated. Event-related potential (ERP) recordings on the scalp offer promise for this issue because they allow brain responses to complex visual stimuli to be relatively well isolated from other sensory, cognitive and motor processes. ERP studies in 5- to 16-year-old children report large age-related changes in amplitude, latency (decreases) and topographical distribution of the early visual components, the P1 and the occipito-temporal N170. To test the face specificity of these effects, we recorded high-density ERPs to pictures of faces, cars, and their phase-scrambled versions from 72 children between the ages of 4 and 17, and a group of adults. We found that none of the previously reported age-dependent changes in amplitude, latency or topography of the P1 or N170 were specific to faces. Most importantly, when we controlled for age-related variations of the P1, the N170 appeared remarkably similar in amplitude and topography across development, with much smaller age-related decreases in latencies than previously reported. At all ages the N170 showed equivalent face-sensitivity: it had the same topography and right hemisphere dominance, it was absent for meaningless (scrambled) stimuli, and larger and earlier for faces than cars. The data also illustrate the large amount of inter-individual and inter-trial variance in young children's data, which causes the N170 to merge with a later component, the N250, in grand-averaged data. Based on our observations, we suggest that the previously reported "bi-fid" N170 of young children is in fact the N250. Overall, our data indicate that the electrophysiological markers of face-sensitive perceptual processes are present from 4 years of age and do not appear to change throughout development.
Keywords: ERP; N170; development; face recognition.
Figures



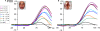

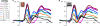







Similar articles
-
ERP evidence for the speed of face categorization in the human brain: Disentangling the contribution of low-level visual cues from face perception.Vision Res. 2011 Jun 21;51(12):1297-311. doi: 10.1016/j.visres.2011.04.003. Epub 2011 Apr 28. Vision Res. 2011. PMID: 21549144
-
Does physical interstimulus variance account for early electrophysiological face sensitive responses in the human brain? Ten lessons on the N170.Neuroimage. 2008 Feb 15;39(4):1959-79. doi: 10.1016/j.neuroimage.2007.10.011. Epub 2007 Oct 22. Neuroimage. 2008. PMID: 18055223 Review.
-
Early lateralization and orientation tuning for face, word, and object processing in the visual cortex.Neuroimage. 2003 Nov;20(3):1609-24. doi: 10.1016/j.neuroimage.2003.07.010. Neuroimage. 2003. PMID: 14642472 Clinical Trial.
-
Early (n170/m170) face-sensitivity despite right lateral occipital brain damage in acquired prosopagnosia.Front Hum Neurosci. 2011 Dec 5;5:138. doi: 10.3389/fnhum.2011.00138. eCollection 2011. Front Hum Neurosci. 2011. PMID: 22275889 Free PMC article.
-
Meta-analysis of face processing event-related potentials in schizophrenia.Biol Psychiatry. 2015 Jan 15;77(2):116-26. doi: 10.1016/j.biopsych.2014.04.015. Epub 2014 May 5. Biol Psychiatry. 2015. PMID: 24923618 Review.
Cited by
-
The development of multisensory integration in high-functioning autism: high-density electrical mapping and psychophysical measures reveal impairments in the processing of audiovisual inputs.Cereb Cortex. 2013 Jun;23(6):1329-41. doi: 10.1093/cercor/bhs109. Epub 2012 May 24. Cereb Cortex. 2013. PMID: 22628458 Free PMC article.
-
Contribution of Neuroimaging Studies to Understanding Development of Human Cognitive Brain Functions.Front Hum Neurosci. 2016 Sep 15;10:464. doi: 10.3389/fnhum.2016.00464. eCollection 2016. Front Hum Neurosci. 2016. PMID: 27695409 Free PMC article. Review.
-
A face detection bias for horizontal orientations develops in middle childhood.Front Psychol. 2015 Jun 8;6:772. doi: 10.3389/fpsyg.2015.00772. eCollection 2015. Front Psychol. 2015. PMID: 26106349 Free PMC article.
-
Charting development of ERP components on face-categorization: Results from a large longitudinal sample of infants.Dev Cogn Neurosci. 2020 Oct;45:100840. doi: 10.1016/j.dcn.2020.100840. Epub 2020 Aug 16. Dev Cogn Neurosci. 2020. PMID: 32877890 Free PMC article.
-
Word selectivity in high-level visual cortex and reading skill.Dev Cogn Neurosci. 2019 Apr;36:100593. doi: 10.1016/j.dcn.2018.09.003. Epub 2018 Sep 29. Dev Cogn Neurosci. 2019. PMID: 30318344 Free PMC article.
References
LinkOut - more resources
Full Text Sources

