Determinants Present in the Receptor Carboxy Tail Are Responsible for Differences in Subtype-Specific Coupling of beta-Adrenergic Receptors to Phosphoinositide 3-Kinase
- PMID: 20130777
- PMCID: PMC2809356
- DOI: 10.1155/2009/959168
Determinants Present in the Receptor Carboxy Tail Are Responsible for Differences in Subtype-Specific Coupling of beta-Adrenergic Receptors to Phosphoinositide 3-Kinase
Abstract
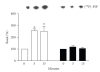
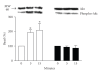
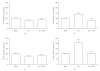
An agonist-occupied beta(2)-adrenergic receptor (beta(2)-AR) recruits G protein receptor kinase-2 (GRK2) which is recruited to the membrane. Thus, the physical proximity of activated beta(2)-AR and PI-3K allows the activation of the latter. In contrast, it has been observed that the beta(1)-AR is unable to activate the PI-3K/Akt pathway. We hypothesized that the difference might be due to molecular determinants present in the carboxy termini of the two beta-AR subtypes. Using transiently transfected HEK 293 cells expressing either beta(1)- or beta(2)-AR, we also observed that in presence of an agonist, beta(2)-AR, but not beta(1)-AR, is able to activate the PI-3K/Akt pathway. Switching the seventh transmembrane domain and the carboxy tail between the two receptors reverses this phenotype; that is, beta(1) x beta(2)-AR can activate the PI-3K/Akt pathway whereas beta(2) x beta(1)-AR cannot. Pretreatment with pertussis toxin abolished the activation of PI-3K by beta(2)- or beta(1) x beta(2)-AR stimulation. Ligand-mediated internalization of the beta(2)-AR induced by a 15-minute stimulation with agonist was abolished in the presence of a dominant negative of PI-3K or following pertussis toxin pretreatment. These results indicate that the subtype-specific differences in the coupling to PI-3K/Akt pathway are due to molecular determinants present in the carboxy tail of the receptor and further that beta(2)-AR activates PI-3K via a pertussis toxin-sensitive mechanism.
Figures





Similar articles
-
The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3'-kinase.Circ Res. 2000 Dec 8;87(12):1172-9. doi: 10.1161/01.res.87.12.1172. Circ Res. 2000. PMID: 11110775
-
Gi-mediated translocation of GLUT4 is independent of p85/p110alpha and p110gamma phosphoinositide 3-kinases but might involve the activation of Akt kinase.Biochem J. 2000 Feb 1;345 Pt 3(Pt 3):543-55. Biochem J. 2000. PMID: 10642513 Free PMC article.
-
Beta 2-adrenergic receptor signaling acts via NO release to mediate ACh-induced activation of ATP-sensitive K+ current in cat atrial myocytes.J Gen Physiol. 2002 Jan;119(1):69-82. doi: 10.1085/jgp.119.1.69. J Gen Physiol. 2002. PMID: 11773239 Free PMC article.
-
Distinct beta-adrenergic receptor subtype signaling in the heart and their pathophysiological relevance.Sheng Li Xue Bao. 2004 Feb 25;56(1):1-15. Sheng Li Xue Bao. 2004. PMID: 14985822 Review.
-
Recent advances in cardiac beta(2)-adrenergic signal transduction.Circ Res. 1999 Nov 26;85(11):1092-100. doi: 10.1161/01.res.85.11.1092. Circ Res. 1999. PMID: 10571541 Review.
References
-
- Chesley A, Lundberg MS, Asai T, et al. The β 2-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through Gi-dependent coupling to phosphatidylinositol 3′-kinase. Circulation Research. 2000;87(12):1172–1179. - PubMed
-
- Naga Prasad SV, Barak LS, Rapacciuolo A, Caron MG, Rockman HA. Agonist-dependent recruitment of phosphoinositide 3-kinase to the membrane by β-adrenergic receptor kinase 1: a role in receptor sequestration. The Journal of Biological Chemistry. 2001;276(22):18953–18959. - PubMed
-
- Xiao R-P, Zhu W, Zheng M, et al. Subtype-specific β-adrenoceptor signaling pathways in the heart and their potential clinical implications. Trends in Pharmacological Sciences. 2004;25(7):358–365. - PubMed
-
- Barak LS, Wilbanks AM, Caron MG. Constitutive desensitization: a new paradigm for g protein-coupled receptor regulation. ASSAY and Drug Development Technologies. 2003;1(2):339–346. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

