Anti-EphA2 Antibodies with Distinct In Vitro Properties Have Equal In Vivo Efficacy in Pancreatic Cancer
- PMID: 20130824
- PMCID: PMC2814375
- DOI: 10.1155/2009/951917
Anti-EphA2 Antibodies with Distinct In Vitro Properties Have Equal In Vivo Efficacy in Pancreatic Cancer
Abstract
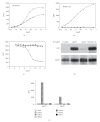
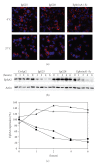
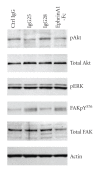
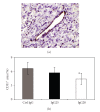
The EphA2 receptor tyrosine kinase is overexpressed in a variety of human epithelial cancers and is a determinant of malignant cellular behavior in pancreatic adenocarcinoma cells. Moreover, it is expressed in tumor endothelium and its activation promotes angiogenesis. To better clarify the therapeutic potential of monoclonal antibodies (mAbs) directed to the EphA2 receptor, we generated a large number of mAbs by differential screening of phage-Ab libraries by oligonucleotide microarray technology and implemented a strategy for the rapid identification of antibodies with the desired properties. We selected two high-affinity and highly specific EphA2 monoclonal antibodies with different in vitro properties on the human pancreatic tumor cell line MiaPaCa2. One is a potent EphA2-agonistic antibody, IgG25, that promotes receptor endocytosis and subsequent degradation, and the second is a ligand antagonist, IgG28, that blocks the binding to ephrin A1 and is cross-reactive with the mouse EphA2 receptor. We measured the effect of antibody treatment on the growth of MiaPaCa2 cells orthotopically transplanted in nude mice. Both IgG25 and IgG28 had strong antitumor and antimetastatic efficacy. In vivo treatment with IgG25 determined the reduction of the EphA2 protein levels in the tumor and the phosphorylation of FAK on Tyr576 while administration of IgG28 caused a decrease in tumor vascularization as measured by immunohistochemical analysis of CD31 in tumor sections. These data show that in a pancreatic cancer model comparable therapeutic efficacy is obtained either by promoting receptor degradation or by blocking receptor activation.
Figures


Similar articles
-
EphA2: a determinant of malignant cellular behavior and a potential therapeutic target in pancreatic adenocarcinoma.Oncogene. 2004 Feb 19;23(7):1448-56. doi: 10.1038/sj.onc.1207247. Oncogene. 2004. Retraction in: Oncogene. 2023 Mar;42(12):938. doi: 10.1038/s41388-023-02626-5. PMID: 14973554 Retracted.
-
Effectiveness of anti-erythropoietin producing Hepatocellular receptor Type-A2 antibody in pancreatic cancer treatment.Heliyon. 2023 Nov 4;9(11):e21774. doi: 10.1016/j.heliyon.2023.e21774. eCollection 2023 Nov. Heliyon. 2023. PMID: 38034633 Free PMC article.
-
PET-Guided Evaluation and Optimization of Internalized Antibody-Drug Conjugates Targeting Erythropoietin-Producing Hepatoma A2 Receptor.J Nucl Med. 2017 Nov;58(11):1838-1844. doi: 10.2967/jnumed.117.192245. Epub 2017 May 25. J Nucl Med. 2017. PMID: 28546337 Free PMC article.
-
The EphA2 and cancer connection: potential for immune-based interventions.Mol Biol Rep. 2020 Oct;47(10):8037-8048. doi: 10.1007/s11033-020-05767-y. Epub 2020 Sep 29. Mol Biol Rep. 2020. PMID: 32990903 Review.
-
Diverse roles for the Eph family of receptor tyrosine kinases in carcinogenesis.Microsc Res Tech. 2002 Oct 1;59(1):58-67. doi: 10.1002/jemt.10177. Microsc Res Tech. 2002. PMID: 12242697 Review.
Cited by
-
The role of EphA7 in different tumors.Clin Transl Oncol. 2022 Jul;24(7):1274-1289. doi: 10.1007/s12094-022-02783-1. Epub 2022 Feb 2. Clin Transl Oncol. 2022. PMID: 35112312 Review.
-
The EPH/Ephrin System in Pancreatic Ductal Adenocarcinoma (PDAC): From Pathogenesis to Treatment.Int J Mol Sci. 2023 Feb 3;24(3):3015. doi: 10.3390/ijms24033015. Int J Mol Sci. 2023. PMID: 36769332 Free PMC article. Review.
-
Effective Tumor Targeting by EphA2-Agonist-Biotin-Streptavidin Conjugates.Molecules. 2021 Jun 17;26(12):3687. doi: 10.3390/molecules26123687. Molecules. 2021. PMID: 34204178 Free PMC article.
-
Targeting the EphA2 pathway: could it be the way for bone sarcomas?Cell Commun Signal. 2024 Sep 9;22(1):433. doi: 10.1186/s12964-024-01811-7. Cell Commun Signal. 2024. PMID: 39252029 Free PMC article. Review.
-
Eph Receptors in Cancer.Biomedicines. 2023 Jan 23;11(2):315. doi: 10.3390/biomedicines11020315. Biomedicines. 2023. PMID: 36830852 Free PMC article. Review.
References
-
- Lackmann M, Boyd AW. Eph, a protein family coming of age: more confusion, insight, or complexity? Science Signaling. 2008;13:1–16. - PubMed
-
- Pasquale EB. Eph receptor signaling casts a wide net on cell behavior. Nature Reviews. 2005;6:462–475. - PubMed
-
- Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133(1):38–52. - PubMed
-
- Merlos-Suárez A, Batlle E. Eph-ephrin signaling in adult tissues and cancer. Current Opinion in Cell Biology. 2008;20:194–200. - PubMed
-
- Ireton RC, Chen J. EphA2 receptor tyrosine kinase as a promising target for cancer therapeutics. Current Cancer Drug Targets. 2005;5(3):149–157. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

