A dual-labeled knottin peptide for PET and near-infrared fluorescence imaging of integrin expression in living subjects
- PMID: 20131753
- PMCID: PMC3004996
- DOI: 10.1021/bc9003102
A dual-labeled knottin peptide for PET and near-infrared fluorescence imaging of integrin expression in living subjects
Abstract
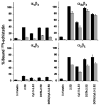
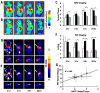
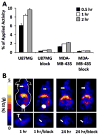
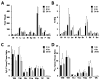
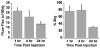
Previously, we used directed evolution to engineer mutants of the Ecballium elaterium trypsin inhibitor (EETI-II) knottin that bind to αvβ3 and αvβ5 integrin receptors with low nanomolar affinity, and showed that Cy5.5- or (64)Cu-DOTA-labeled knottin peptides could be used to image integrin expression in mouse tumor models using near-infrared fluorescence (NIRF) imaging or positron emission tomography (PET). Here, we report the development of a dual-labeled knottin peptide conjugated to both NIRF and PET imaging agents for multimodality imaging in living subjects. We created an orthogonally protected peptide-based linker for stoichiometric coupling of (64)Cu-DOTA and Cy5.5 onto the knottin N-terminus and confirmed that conjugation did not affect binding to αvβ3 and αvβ5 integrins. NIRF and PET imaging studies in tumor xenograft models showed that Cy5.5 conjugation significantly increased kidney uptake and retention compared to the knottin peptide labeled with (64)Cu-DOTA alone. In the tumor, the dual-labeled (64)Cu-DOTA/Cy5.5 knottin peptide showed decreased wash-out leading to significantly better retention (p < 0.05) compared to the (64)Cu-DOTA-labeled knottin peptide. Tumor uptake was significantly reduced (p < 0.05) when the dual-labeled knottin peptide was coinjected with an excess of unlabeled competitor and when tested in a tumor model with lower levels of integrin expression. Finally, plots of tumor-to-background tissue ratios for Cy5.5 versus (64)Cu uptake were well-correlated over several time points post injection, demonstrating pharmacokinetic cross validation of imaging labels. This dual-modality NIRF/PET imaging agent is promising for further development in clinical applications where high sensitivity and high resolution are desired, such as detection of tumors located deep within the body and image-guided surgical resection.
Figures


References
-
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. - PubMed
-
- Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alphav beta3 for angiogenesis. Science. 1994;264:569–571. - PubMed
-
- Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct alphav integrins. Science. 1995;270:1500–1502. - PubMed
-
- Alghisi GC, Ruegg C. Vascular integrins in tumor angiogenesis: mediators and therapeutic targets. Endothelium. 2006;13:113–135. - PubMed
-
- Mizejewski GJ. Role of integrins in cancer: survey of expression patterns. Proc Soc Exp Biol Med. 1999;222:124–138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

