Interactions between buprenorphine and antiretrovirals: nucleos(t)ide reverse transcriptase inhibitors (NRTI) didanosine, lamivudine, and tenofovir
- PMID: 20132118
- PMCID: PMC3723405
- DOI: 10.1111/j.1521-0391.2009.00004.x
Interactions between buprenorphine and antiretrovirals: nucleos(t)ide reverse transcriptase inhibitors (NRTI) didanosine, lamivudine, and tenofovir
Abstract
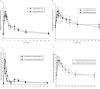
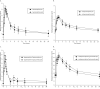
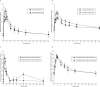
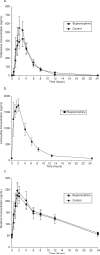
To improve outcomes among injection drug users with HIV and/or chronic hepatitis B, it is important to identify drug interactions between antiretroviral and opiate therapies. We report the results of a study designed to examine the interaction between buprenorphine and the nucleos(t)ide reverse transcriptase inhibitors (NRTI) didanosine (ddI), lamivudine (3TC), and tenofovir (TDF). Opioid-dependent, buprenorphine/naloxone-maintained, HIV-negative volunteers (n = 27) participated in two 24-hour sessions to determine (1) pharmacokinetics of buprenorphine alone and (2) pharmacokinetics of both buprenorphine and either ddI, 3TC, or TDF. Among buprenorphine/naloxone-maintained study participants, no significant changes in buprenorphine pharmacokinetics were observed following ddI, 3TC, or TDF administration. Buprenorphine had no significant effect on NRTI concentrations. Concomitant use of buprenorphine with ddI, 3TC, or TDF results in neither a significant pharmacokinetic nor pharmacodynamic interaction.
Figures
Similar articles
-
Pilot pharmacokinetic study of human immunodeficiency virus-infected patients receiving tenofovir disoproxil fumarate (TDF): investigation of systemic and intracellular interactions between TDF and abacavir, lamivudine, or lopinavir-ritonavir.Antimicrob Agents Chemother. 2009 May;53(5):1937-43. doi: 10.1128/AAC.01064-08. Epub 2009 Mar 9. Antimicrob Agents Chemother. 2009. PMID: 19273671 Free PMC article. Clinical Trial.
-
[Immunological effectiveness of the nucleoside/nucleotide analog combinations, tenofovir + lamivudine, didanosine + lamivudine and tenofovir + didanosine, in patients with HIV infection and sustained viral suppression].Enferm Infecc Microbiol Clin. 2006 Aug-Sep;24(7):426-30. doi: 10.1157/13091779. Enferm Infecc Microbiol Clin. 2006. PMID: 16956530 Spanish.
-
Intracellular pharmacokinetics of tenofovir diphosphate, carbovir triphosphate, and lamivudine triphosphate in patients receiving triple-nucleoside regimens.J Acquir Immune Defic Syndr. 2005 Aug 1;39(4):406-11. doi: 10.1097/01.qai.0000167155.44980.e8. J Acquir Immune Defic Syndr. 2005. PMID: 16010161
-
Tenofovir plus didanosine as Nrti backbone in HIV-infected subjects.Curr Med Chem. 2006;13(23):2789-93. doi: 10.2174/092986706778521931. Curr Med Chem. 2006. PMID: 17073629 Review.
-
[Tenofovir: pharmacology and interactions].Enferm Infecc Microbiol Clin. 2008 Jun;26 Suppl 8:2-6. doi: 10.1157/13126265. Enferm Infecc Microbiol Clin. 2008. PMID: 19195431 Review. Spanish.
Cited by
-
A review of pharmacological interactions between HIV or hepatitis C virus medications and opioid agonist therapy: implications and management for clinical practice.Expert Rev Clin Pharmacol. 2013 May;6(3):249-69. doi: 10.1586/ecp.13.18. Expert Rev Clin Pharmacol. 2013. PMID: 23656339 Free PMC article. Review.
-
Non-injection drug use and HIV disease progression in the era of combination antiretroviral therapy.J Subst Abuse Treat. 2011 Jun;40(4):386-96. doi: 10.1016/j.jsat.2011.01.001. Epub 2011 Feb 24. J Subst Abuse Treat. 2011. PMID: 21353444 Free PMC article.
-
New developments in the management of opioid dependence: focus on sublingual buprenorphine-naloxone.Subst Abuse Rehabil. 2015 Jan 6;6:1-14. doi: 10.2147/SAR.S45585. eCollection 2015. Subst Abuse Rehabil. 2015. PMID: 25610012 Free PMC article. Review.
-
Gender differences in pharmacokinetics of maintenance dosed buprenorphine.Drug Alcohol Depend. 2011 Nov 1;118(2-3):479-83. doi: 10.1016/j.drugalcdep.2011.03.024. Epub 2011 Apr 23. Drug Alcohol Depend. 2011. PMID: 21515002 Free PMC article.
-
Therapeutic drug monitoring of protease inhibitors and efavirenz in HIV-infected individuals with active substance-related disorders.Ther Drug Monit. 2011 Jun;33(3):309-14. doi: 10.1097/FTD.0b013e31821d3adb. Ther Drug Monit. 2011. PMID: 21544014 Free PMC article. Clinical Trial.
References
-
- UNAIDS [April 15, 2009]; Available at: http://www.unaids.org/en/knowledgecentre/hivdata/epidemiology/epislides.asp.
-
- Peters MG. Diagnosis and management of hepatitis B virus and HIV coinfection. Top HIV Med. 2007;15:163–166. - PubMed
-
- Howard AA, Arnsten JH, Lo Y, et al. A prospective study of adherence and viral load in a large multi-center cohort of HIV-infected women. AIDS. 2002;16:2175–2182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

