Interactions between buprenorphine and antiretrovirals: nucleos(t)ide reverse transcriptase inhibitors (NRTI) didanosine, lamivudine, and tenofovir
- PMID: 20132118
- PMCID: PMC3723405
- DOI: 10.1111/j.1521-0391.2009.00004.x
Interactions between buprenorphine and antiretrovirals: nucleos(t)ide reverse transcriptase inhibitors (NRTI) didanosine, lamivudine, and tenofovir
Abstract
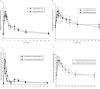
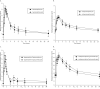
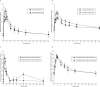
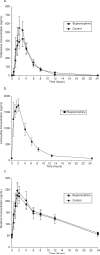
To improve outcomes among injection drug users with HIV and/or chronic hepatitis B, it is important to identify drug interactions between antiretroviral and opiate therapies. We report the results of a study designed to examine the interaction between buprenorphine and the nucleos(t)ide reverse transcriptase inhibitors (NRTI) didanosine (ddI), lamivudine (3TC), and tenofovir (TDF). Opioid-dependent, buprenorphine/naloxone-maintained, HIV-negative volunteers (n = 27) participated in two 24-hour sessions to determine (1) pharmacokinetics of buprenorphine alone and (2) pharmacokinetics of both buprenorphine and either ddI, 3TC, or TDF. Among buprenorphine/naloxone-maintained study participants, no significant changes in buprenorphine pharmacokinetics were observed following ddI, 3TC, or TDF administration. Buprenorphine had no significant effect on NRTI concentrations. Concomitant use of buprenorphine with ddI, 3TC, or TDF results in neither a significant pharmacokinetic nor pharmacodynamic interaction.
Figures
References
-
- UNAIDS [April 15, 2009]; Available at: http://www.unaids.org/en/knowledgecentre/hivdata/epidemiology/epislides.asp.
-
- Peters MG. Diagnosis and management of hepatitis B virus and HIV coinfection. Top HIV Med. 2007;15:163–166. - PubMed
-
- Howard AA, Arnsten JH, Lo Y, et al. A prospective study of adherence and viral load in a large multi-center cohort of HIV-infected women. AIDS. 2002;16:2175–2182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

