Ligand-directed signalling at beta-adrenoceptors
- PMID: 20132209
- PMCID: PMC2839261
- DOI: 10.1111/j.1476-5381.2009.00602.x
Ligand-directed signalling at beta-adrenoceptors
Abstract
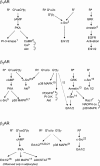
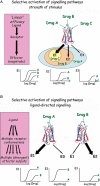
beta-Adrenoceptors (ARs) classically mediate responses to the endogenous ligands adrenaline and noradrenaline by coupling to Gsalpha and stimulating cAMP production; however, drugs designed as beta-AR agonists or antagonists can activate alternative cell signalling pathways, with the potential to influence clinical efficacy. Furthermore, drugs acting at beta-ARs have differential capacity for pathway activation, described as stimulus trafficking, biased agonism, functional selectivity or ligand-directed signalling. These terms refer to responses where drug A has higher efficacy than drug B for one signalling pathway, but a lower efficacy than drug B for a second pathway. The accepted explanation for such responses is that drugs A and B have the capacity to induce or stabilize distinct active conformations of the receptor that in turn display altered coupling efficiency to different effectors. This is consistent with biophysical studies showing that drugs can indeed promote distinct conformational states. Agonists acting at beta-ARs display ligand-directed signalling, but many drugs acting as cAMP antagonists are also able to activate signalling pathways central to cell survival and proliferation or cell death. The observed complexity of drug activity at beta-ARs, prototypical G protein-coupled receptors, necessitates rethinking of the approaches used for screening and characterization of novel therapeutic agents. Most studies of ligand-directed signalling employ recombinant cell systems with high receptor abundance. While such systems are valid for examining upstream signalling events, such as receptor conformational changes and G protein activation, they are less robust when comparing downstream signalling outputs as these are likely to be affected by complex pathway interactions.
Figures
Similar articles
-
The rush to adrenaline: drugs in sport acting on the beta-adrenergic system.Br J Pharmacol. 2008 Jun;154(3):584-97. doi: 10.1038/bjp.2008.164. Br J Pharmacol. 2008. PMID: 18500380 Free PMC article. Review.
-
Ligand-directed trafficking of receptor stimulus.Pharmacol Rep. 2014 Dec;66(6):1011-21. doi: 10.1016/j.pharep.2014.06.006. Epub 2014 Jun 26. Pharmacol Rep. 2014. PMID: 25443729 Review.
-
Selective activation of beta3-adrenoceptors by octopamine: comparative studies in mammalian fat cells.Naunyn Schmiedebergs Arch Pharmacol. 1999 Apr;359(4):310-21. doi: 10.1007/pl00005357. Naunyn Schmiedebergs Arch Pharmacol. 1999. PMID: 10344530
-
Atypical pharmacologies at beta-adrenoceptors.Br J Pharmacol. 2008 Oct;155(3):285-7. doi: 10.1038/bjp.2008.293. Epub 2008 Jul 21. Br J Pharmacol. 2008. PMID: 18641673 Free PMC article.
-
Divergent agonist selectivity in activating β1- and β2-adrenoceptors for G-protein and arrestin coupling.Biochem J. 2011 Aug 15;438(1):191-202. doi: 10.1042/BJ20110374. Biochem J. 2011. PMID: 21561432
Cited by
-
Advances in receptor conformation research: the quest for functionally selective conformations focusing on the β2-adrenoceptor.Br J Pharmacol. 2015 Dec;172(23):5477-88. doi: 10.1111/bph.13049. Epub 2015 Feb 27. Br J Pharmacol. 2015. PMID: 25537131 Free PMC article. Review.
-
Role of β-blockers in Preventing Heart Failure and Major Adverse Cardiac Events Post Myocardial Infarction.Curr Cardiol Rev. 2023;19(4):e110123212591. doi: 10.2174/1573403X19666230111143901. Curr Cardiol Rev. 2023. PMID: 36635926 Free PMC article. Review.
-
Association of beta-adrenergic receptor polymorphisms and mortality in carvedilol-treated chronic heart-failure patients.Br J Clin Pharmacol. 2011 Apr;71(4):556-65. doi: 10.1111/j.1365-2125.2010.03868.x. Br J Clin Pharmacol. 2011. PMID: 21395649 Free PMC article.
-
Angiopoietin-like 4 (Angptl4) protein is a physiological mediator of intracellular lipolysis in murine adipocytes.J Biol Chem. 2012 Mar 9;287(11):8444-56. doi: 10.1074/jbc.M111.294124. Epub 2012 Jan 19. J Biol Chem. 2012. PMID: 22267746 Free PMC article.
-
Trafficking of β-Adrenergic Receptors: Implications in Intracellular Receptor Signaling.Prog Mol Biol Transl Sci. 2015;132:151-88. doi: 10.1016/bs.pmbts.2015.03.008. Epub 2015 Apr 29. Prog Mol Biol Transl Sci. 2015. PMID: 26055058 Free PMC article. Review.
References
-
- Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of β-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004;279:35518–35525. - PubMed
-
- Ali MS, Sayeski PP, Dirksen LB, Hayzer DJ, Marrero MB, Bernstein KE. Dependence on the motif YIPP for the physical association of Jak2 kinase with the intracellular carboxyl tail of the angiotensin II AT1 receptor. J Biol Chem. 1997;272:23382–23388. - PubMed
-
- Angelone T, Filice E, Quintieri AM, Imbrogno S, Recchia A, Pulera E, et al. β3-Adrenoceptors modulate left ventricular relaxation in the rat heart via the NO–cGMP–PKG pathway. Acta Physiol (Oxf) 2008;193:229–239. - PubMed
-
- Audet M, Bouvier M. Insights into signaling from the β2-adrenergic receptor structure. Nat Chem Biol. 2008;4:397–403. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

