Mechanisms involved in the antinociception induced by systemic administration of guanosine in mice
- PMID: 20132210
- PMCID: PMC2848929
- DOI: 10.1111/j.1476-5381.2009.00597.x
Mechanisms involved in the antinociception induced by systemic administration of guanosine in mice
Abstract
Background and purpose: It is well known that adenine-based purines exert multiple effects on pain transmission. However, less attention has been given to the potential effects of guanine-based purines on pain transmission. The aim of this study was to investigate the effects of intraperitoneal (i.p.) and oral (p.o.) administration of guanosine on mice pain models. Additionally, investigation into the mechanisms of action of guanosine, its potential toxicity and cerebrospinal fluid (CSF) purine levels were also assessed.
Experimental approach: Mice received an i.p. or p.o. administration of vehicle (0.1 mM NaOH) or guanosine (up to 240 mg x kg(-1)) and were evaluated in several pain models.
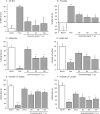
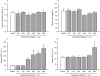
Key results: Guanosine produced dose-dependent antinociceptive effects in the hot-plate, glutamate, capsaicin, formalin and acetic acid models, but it was ineffective in the tail-flick test. Additionally, guanosine produced a significant inhibition of biting behaviour induced by i.t. injection of glutamate, AMPA, kainate and trans-ACPD, but not against NMDA, substance P or capsaicin. The antinociceptive effects of guanosine were prevented by selective and non-selective adenosine receptor antagonists. Systemic administration of guanosine (120 mg x kg(-1)) induced an approximately sevenfold increase on CSF guanosine levels. Guanosine prevented the increase on spinal cord glutamate uptake induced by intraplantar capsaicin.
Conclusions and implications: This study provides new evidence on the mechanism of action of the antinociceptive effects after systemic administration of guanosine. These effects seem to be related to the modulation of adenosine A(1) and A(2A) receptors and non-NMDA glutamate receptors.
Figures
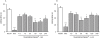


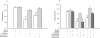


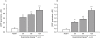
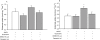
References
-
- Abacioglu N, Tunçtan B, Cakici I, Akbulut E, Uludağ O, Kanzik I. The role of L-arginine/nitric oxide pathway in the antinociceptive activity of pyridoxine in mouse. Arzneimittelforschung. 2001;51:832–838. - PubMed
-
- Baron BM, Dudley MW, McCarty DR, Miller FP, Reynolds IJ, Schmidt CJ. Guanine nucleotides are competitive inhibitors of N-Methyl-D-Aspartate at its receptor site both in vitro and in vivo. J Pharmacol Exp Ther. 1989;250:162–169. - PubMed
-
- Bastia E, Varani K, Monopoli A, Bertorelli R. Effects of A(1) and A(2A) adenosine receptor ligands in mouse acute models of pain. Neurosci Lett. 2002;328:241–244. - PubMed
-
- Beirith A, Santos ARS, Calixto JB. Mechanisms underlying the nociception and paw oedema caused by injection of glutamate into the mouse paw. Brain Res. 2002;924:219–228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

