Microglial C5aR (CD88) expression correlates with amyloid-beta deposition in murine models of Alzheimer's disease
- PMID: 20132482
- PMCID: PMC2921960
- DOI: 10.1111/j.1471-4159.2010.06595.x
Microglial C5aR (CD88) expression correlates with amyloid-beta deposition in murine models of Alzheimer's disease
Abstract
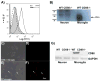
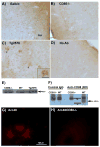
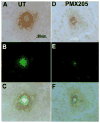
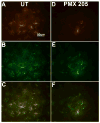
Alzheimer's disease (AD), a progressive neurodegenerative disease characterized by the accumulation of amyloid-beta protein and neuronal loss, is the leading cause of age-related dementia in the world today. The disease is also associated with neuroinflammation, robust activation of astrocytes and microglia, and evidence of activation of the complement system, localized with both fibrillar amyloid-beta (fAbeta) plaques and tangles. The observations are consistent with a complement-dependent component of AD progression. We have previously shown that inhibition of the major complement receptor for C5a (CD88) with the antagonist PMX205 results in a significant reduction in pathology in two mouse models of AD. To further characterize the role of complement in AD-related neuroinflammation, we examined the age- and disease-associated expression of CD88 in brain of transgenic mouse models of AD and the influence of PMX205 on the presence of various complement activation products using flow cytometry, western blot, and immunohistochemistry. CD88 was found to be up-regulated in microglia, in the immediate vicinity of amyloid plaques. While thioflavine plaque load and glial recruitment is significantly reduced after treatment with PMX205, C1q remains co-localized with fAbeta plaques and C3 is still expressed by the recruited astrocytes. Thus, with PMX205, potentially beneficial activities of these early complement components may remain intact, while detrimental activities resulting from C5a-CD88 interaction are inhibited. This further supports the targeted inhibition of specific complement mediated activities as an approach for AD therapy.
Figures




References
-
- Afagh A, Cummings BJ, Cribbs DH, Cotman CW, Tenner AJ. Localization and cell association of C1q in Alzheimer’s disease brain. Exp Neurol. 1996;138:22–32. - PubMed
-
- Bao L, Osawe I, Puri T, Lambris JD, Haas M, Quigg RJ. C5a promotes development of experimental lupus nephritis which can be blocked with a specific receptor antagonist. Eur J Immunol. 2005;35:2496–2506. - PubMed
-
- Bonifati DM, Kishore U. Role of complement in neurodegeneration and neuroinflammation. Mol Immunol. 2007;44:999–1010. - PubMed
-
- Botto M, Dell’agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, Loos M, Pandolfi PP, Walport MJ. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

