A novel, high-efficiency cellular model of fibrillar alpha-synuclein inclusions and the examination of mutations that inhibit amyloid formation
- PMID: 20132485
- PMCID: PMC2907888
- DOI: 10.1111/j.1471-4159.2010.06592.x
A novel, high-efficiency cellular model of fibrillar alpha-synuclein inclusions and the examination of mutations that inhibit amyloid formation
Abstract
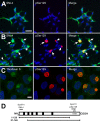
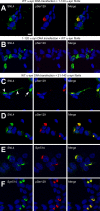
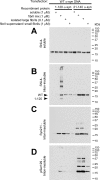
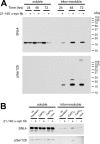
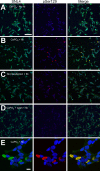
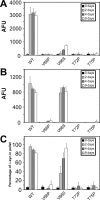
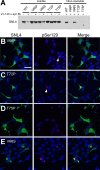
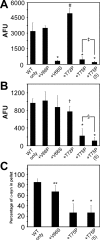
Intracytoplasmic alpha-synuclein (alpha-syn) amyloidogenic inclusions are a major pathological feature of Parkinson's disease, dementia with Lewy body disease and multiple systems atrophy. The mechanisms involved in the formation and inhibition of these aggregates are areas of intense investigation. The present study characterizes a novel cellular model for the study of alpha-syn aggregation, incorporating nucleation-dependent aggregation and a new function for calcium phosphate precipitation. Cultured cells were readily induced to develop large, cytoplasmic alpha-syn filamentous aggregates that were hyperphosphorylated, often ubiquitinated and thioflavin positive. These cellular aggregates formed in the majority of transfected cells and recruited approximately half of endogenously expressed alpha-syn. Using this system, we examined single-point mutations that inhibit alpha-syn amyloid formation in vitro. Three mutations (V66P, T72P and T75P) significantly hindered alpha-syn aggregation in this cell model. The T75P mutant, which could abrogate amyloid formation of wild-type alpha-syn in vitro, did not prevent wild-type alpha-syn cellular aggregates. These studies suggest that the propensity of alpha-syn to form cellular aggregates may be more pronounced than in isolated in vitro studies. This novel high-efficiency cellular model of alpha-syn aggregation is a valuable system that may be used to further understand alpha-syn aggregation and allow for the generation of future therapeutics.
Figures
Similar articles
-
Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells.Proc Natl Acad Sci U S A. 2009 Nov 24;106(47):20051-6. doi: 10.1073/pnas.0908005106. Epub 2009 Nov 5. Proc Natl Acad Sci U S A. 2009. PMID: 19892735 Free PMC article.
-
Distinct Effects of Familial Parkinson's Disease-Associated Mutations on α-Synuclein Phase Separation and Amyloid Aggregation.Biomolecules. 2023 Apr 23;13(5):726. doi: 10.3390/biom13050726. Biomolecules. 2023. PMID: 37238596 Free PMC article.
-
Induction of intracellular tau aggregation is promoted by α-synuclein seeds and provides novel insights into the hyperphosphorylation of tau.J Neurosci. 2011 May 25;31(21):7604-18. doi: 10.1523/JNEUROSCI.0297-11.2011. J Neurosci. 2011. PMID: 21613474 Free PMC article.
-
Neuropathology of synuclein aggregates.J Neurosci Res. 2000 Jul 15;61(2):121-7. doi: 10.1002/1097-4547(20000715)61:2<121::AID-JNR1>3.0.CO;2-4. J Neurosci Res. 2000. PMID: 10878583 Review.
-
α-Synuclein misfolding and aggregation: Implications in Parkinson's disease pathogenesis.Biochim Biophys Acta Proteins Proteom. 2019 Oct;1867(10):890-908. doi: 10.1016/j.bbapap.2019.03.001. Epub 2019 Mar 7. Biochim Biophys Acta Proteins Proteom. 2019. PMID: 30853581 Review.
Cited by
-
Robust Central Nervous System Pathology in Transgenic Mice following Peripheral Injection of α-Synuclein Fibrils.J Virol. 2017 Jan 3;91(2):e02095-16. doi: 10.1128/JVI.02095-16. Print 2017 Jan 15. J Virol. 2017. PMID: 27852849 Free PMC article.
-
Intrastriatal injection of α-synuclein can lead to widespread synucleinopathy independent of neuroanatomic connectivity.Mol Neurodegener. 2017 May 29;12(1):40. doi: 10.1186/s13024-017-0182-z. Mol Neurodegener. 2017. PMID: 28552073 Free PMC article.
-
O-GlcNAc forces an α-synuclein amyloid strain with notably diminished seeding and pathology.Nat Chem Biol. 2024 May;20(5):646-655. doi: 10.1038/s41589-024-01551-2. Epub 2024 Feb 12. Nat Chem Biol. 2024. PMID: 38347213 Free PMC article.
-
Physiological C-terminal truncation of α-synuclein potentiates the prion-like formation of pathological inclusions.J Biol Chem. 2018 Dec 7;293(49):18914-18932. doi: 10.1074/jbc.RA118.005603. Epub 2018 Oct 16. J Biol Chem. 2018. PMID: 30327435 Free PMC article.
-
Effects of Mutations and Post-Translational Modifications on α-Synuclein In Vitro Aggregation.J Mol Biol. 2022 Dec 15;434(23):167859. doi: 10.1016/j.jmb.2022.167859. Epub 2022 Oct 19. J Mol Biol. 2022. PMID: 36270580 Free PMC article. Review.
References
-
- Ahn KJ, Paik SR, Chung KC, Kim J. Amino acid sequence motifs and mechanistic features of the membrane translocation of alpha-synuclein. J. Neurochem. 2006;97:265–279. - PubMed
-
- Anderson JP, Walker DE, Goldstein JM, et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J. Biol. Chem. 2006;281:29739–29752. - PubMed
-
- Ardley HC, Scott GB, Rose SA, Tan NG, Robinson PA. UCH-L1 aggresome formation in response to proteasome impairment indicates a role in inclusion formation in Parkinson's disease. J. Neurochem. 2004;90:379–391. - PubMed
-
- Biere AL, Wood SJ, Wypych J, et al. Parkinson's disease-associated alpha-synuclein is more fibrillogenic than β- and γ-synuclein and cannot cross-seed its homologs. J. Biol. Chem. 2000;275:34574–34579. - PubMed
-
- Bisaglia M, Schievano E, Caporale A, Peggion E, Mammi S. The 11-mer repeats of human alpha-synuclein in vesicle interactions and lipid composition discrimination: a cooperative role. Biopolymers. 2006;84:310–316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

