Origin of the cell nucleus, mitosis and sex: roles of intracellular coevolution
- PMID: 20132544
- PMCID: PMC2837639
- DOI: 10.1186/1745-6150-5-7
Origin of the cell nucleus, mitosis and sex: roles of intracellular coevolution
Abstract
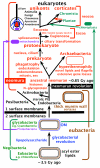
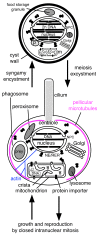
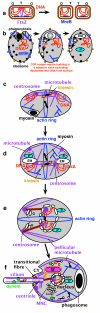
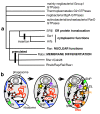
Background: The transition from prokaryotes to eukaryotes was the most radical change in cell organisation since life began, with the largest ever burst of gene duplication and novelty. According to the coevolutionary theory of eukaryote origins, the fundamental innovations were the concerted origins of the endomembrane system and cytoskeleton, subsequently recruited to form the cell nucleus and coevolving mitotic apparatus, with numerous genetic eukaryotic novelties inevitable consequences of this compartmentation and novel DNA segregation mechanism. Physical and mutational mechanisms of origin of the nucleus are seldom considered beyond the long-standing assumption that it involved wrapping pre-existing endomembranes around chromatin. Discussions on the origin of sex typically overlook its association with protozoan entry into dormant walled cysts and the likely simultaneous coevolutionary, not sequential, origin of mitosis and meiosis.
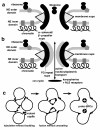
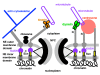
Results: I elucidate nuclear and mitotic coevolution, explaining the origins of dicer and small centromeric RNAs for positionally controlling centromeric heterochromatin, and how 27 major features of the cell nucleus evolved in four logical stages, making both mechanisms and selective advantages explicit: two initial stages (origin of 30 nm chromatin fibres, enabling DNA compaction; and firmer attachment of endomembranes to heterochromatin) protected DNA and nascent RNA from shearing by novel molecular motors mediating vesicle transport, division, and cytoplasmic motility. Then octagonal nuclear pore complexes (NPCs) arguably evolved from COPII coated vesicle proteins trapped in clumps by Ran GTPase-mediated cisternal fusion that generated the fenestrated nuclear envelope, preventing lethal complete cisternal fusion, and allowing passive protein and RNA exchange. Finally, plugging NPC lumens by an FG-nucleoporin meshwork and adopting karyopherins for nucleocytoplasmic exchange conferred compartmentation advantages. These successive changes took place in naked growing cells, probably as indirect consequences of the origin of phagotrophy. The first eukaryote had 1-2 cilia and also walled resting cysts; I outline how encystation may have promoted the origin of meiotic sex. I also explain why many alternative ideas are inadequate.
Conclusion: Nuclear pore complexes are evolutionary chimaeras of endomembrane- and mitosis-related chromatin-associated proteins. The keys to understanding eukaryogenesis are a proper phylogenetic context and understanding organelle coevolution: how innovations in one cell component caused repercussions on others.
Figures
References
-
- Stanier RY. In: Organization and Control in Prokaryotic and Eukaryotic Cells (Society for General Microbiology Symposium no 20) Charles HP, Knight JG, editor. Cambridge: Cambridge University Press; 1970. Some aspects of the biology of cells and their possible evolutionary significance; pp. 1–38.
-
- Cavalier-Smith T. The phagotrophic origin of eukaryotes and phylogenetic classification of Protozoa. Int J Syst Evol Microbiol. 2002;52(Pt 2):297–354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

