Prosaposin down-modulation decreases metastatic prostate cancer cell adhesion, migration, and invasion
- PMID: 20132547
- PMCID: PMC2825248
- DOI: 10.1186/1476-4598-9-30
Prosaposin down-modulation decreases metastatic prostate cancer cell adhesion, migration, and invasion
Abstract
Background: Factors responsible for invasive and metastatic progression of prostate cancer (PCa) remain largely unknown. Previously, we reported cloning of prosaposin (PSAP) and its genomic amplification and/or overexpression in several androgen-independent metastatic PCa cell lines and lymph node metastases. PSAP is the lysosomal precursor of saposins, which serve as activators for lysosomal hydrolases involved in the degradation of ceramide (Cer) and other sphingolipids.
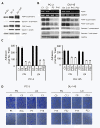
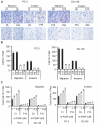
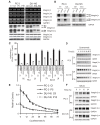
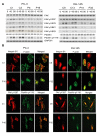
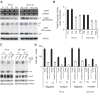
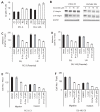
Results: Our current data show that, in metastatic PCa cells, stable down-modulation of PSAP by RNA-interference via a lysosomal proteolysis-dependent pathway decreased beta1A-integrin expression, its cell-surface clustering, and adhesion to basement membrane proteins; led to disassembly of focal adhesion complex; and decreased phosphorylative activity of focal adhesion kinase and its downstream adaptor molecule, paxillin. Cathepsin D (CathD) expression and proteolytic activity, migration, and invasion were also significantly decreased in PSAP knock-down cells. Transient-transfection studies with beta1A integrin- or CathD-siRNA oligos confirmed the cause and effect relationship between PSAP and CathD or PSAP and Cer-beta1A integrin, regulating PCa cell migration and invasion.
Conclusion: Our findings suggest that by a coordinated regulation of Cer levels, CathD and beta1A-integrin expression, and attenuation of "inside-out" integrin-signaling pathway, PSAP is involved in PCa invasion and therefore might be used as a molecular target for PCa therapy.
Figures
Similar articles
-
Amplification and overexpression of prosaposin in prostate cancer.Genes Chromosomes Cancer. 2005 Dec;44(4):351-64. doi: 10.1002/gcc.20249. Genes Chromosomes Cancer. 2005. PMID: 16080200
-
3,4-Methylenedioxy-β-nitrostyrene inhibits adhesion and migration of human triple-negative breast cancer cells by suppressing β1 integrin function and surface protein disulfide isomerase.Biochimie. 2015 Mar;110:81-92. doi: 10.1016/j.biochi.2015.01.006. Epub 2015 Jan 13. Biochimie. 2015. PMID: 25593085
-
Talin1 phosphorylation activates β1 integrins: a novel mechanism to promote prostate cancer bone metastasis.Oncogene. 2015 Apr 2;34(14):1811-21. doi: 10.1038/onc.2014.116. Epub 2014 May 5. Oncogene. 2015. PMID: 24793790 Free PMC article.
-
Focal adhesion kinase controls prostate cancer progression via intrinsic kinase and scaffolding functions.Anticancer Agents Med Chem. 2011 Sep;11(7):607-16. doi: 10.2174/187152011796817646. Anticancer Agents Med Chem. 2011. PMID: 21355844 Review.
-
Dissecting the roles of prosaposin as an emerging therapeutic target for tumors and its underlying mechanisms.Biomed Pharmacother. 2024 Nov;180:117551. doi: 10.1016/j.biopha.2024.117551. Epub 2024 Oct 13. Biomed Pharmacother. 2024. PMID: 39405903 Review.
Cited by
-
Prosaposin, a regulator of estrogen receptor alpha, promotes breast cancer growth.Cancer Sci. 2012 Oct;103(10):1820-5. doi: 10.1111/j.1349-7006.2012.02374.x. Epub 2012 Aug 10. Cancer Sci. 2012. PMID: 22738294 Free PMC article.
-
Total glucosides of paeony inhibits lipopolysaccharide-induced proliferation, migration and invasion in androgen insensitive prostate cancer cells.PLoS One. 2017 Aug 4;12(8):e0182584. doi: 10.1371/journal.pone.0182584. eCollection 2017. PLoS One. 2017. PMID: 28783760 Free PMC article.
-
Putative Biomarkers for Malignant Pleural Mesothelioma Suggested by Proteomic Analysis of Cell Secretome.Cancer Genomics Proteomics. 2020 May-Jun;17(3):225-236. doi: 10.21873/cgp.20183. Cancer Genomics Proteomics. 2020. PMID: 32345664 Free PMC article.
-
Androgen-responsive serum response factor target genes regulate prostate cancer cell migration.Carcinogenesis. 2013 Aug;34(8):1737-46. doi: 10.1093/carcin/bgt126. Epub 2013 Apr 10. Carcinogenesis. 2013. PMID: 23576568 Free PMC article.
-
Serum prosaposin levels are increased in patients with advanced prostate cancer.Prostate. 2012 Feb;72(3):253-69. doi: 10.1002/pros.21427. Epub 2011 May 31. Prostate. 2012. PMID: 21630292 Free PMC article.
References
-
- Kishimoto Y, Hiraiwa M, O'Brien JS. Saposins: Structure, function, distribution and molecular genetics. J Lipid Res. 1992;33:1255–1267. - PubMed
-
- Koochekpour S. PSAP (Prosaposin (variant Gaucher disease and variant metachromatic leukodystrophy)) Atlas Genet Cytogenet Oncol Haematol. 2006. http://AtlasGeneticsOncology.org/Genes/PSAPID42980ch10q22.html
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

