A(3) adenosine receptor activation during reperfusion reduces infarct size through actions on bone marrow-derived cells
- PMID: 20132822
- PMCID: PMC2885516
- DOI: 10.1016/j.yjmcc.2010.01.018
A(3) adenosine receptor activation during reperfusion reduces infarct size through actions on bone marrow-derived cells
Abstract
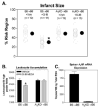
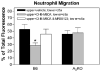
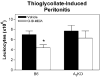
The goal of this study was to examine whether the A(3) adenosine receptor (A(3)AR) agonist Cl-IB-MECA protects against myocardial ischemia/reperfusion injury when administered at the time of reperfusion in an in vivo mouse model of infarction induced by 30min of coronary occlusion and 24h of reperfusion. Treating B6 wild-type with Cl-IB-MECA during the reperfusion phase (100microg/kg i.v. bolus+0.3microg/kg/min subcutaneously via implantation of Alzet mini-osmotic pumps) reduced myocardial infarct size approximately 37% from 50.1+/-2.5% in vehicle-treated mice to 31.6+/-2.8% in Cl-IB-MECA-treated mice, and significantly reduced the number of leukocytes that infiltrated into the ischemic-reperfused myocardium. Cl-IB-MECA did not reduce infarct size or limit leukocyte accumulation in studies using B6 congenic A(3)AR gene "knock-out" mice or in chimeric mice lacking the expression of A(3)ARs in bone marrow (BM)-derived cells. Subsequent mechanistic studies demonstrated that Cl-IB-MECA inhibited migration of mouse neutrophils isolated from BM towards the chemotactic substance c5a in trans-well migration assays, and inhibited leukocyte migration into the peritoneal cavity in a mouse model of thioglycollate-induced peritonitis. We conclude that treating with the A(3)AR agonist Cl-IB-MECA at the time of reperfusion provides effective protection from ischemia/reperfusion injury in the heart through activation of the A(3)AR expressed in BM-derived cells, potentially by suppressing the robust inflammatory reaction that occurs during reperfusion and neutrophil-mediated tissue injury.
Figures


Similar articles
-
Cl-IB-MECA [2-chloro-N6-(3-iodobenzyl)adenosine-5'-N-methylcarboxamide] reduces ischemia/reperfusion injury in mice by activating the A3 adenosine receptor.J Pharmacol Exp Ther. 2006 Dec;319(3):1200-10. doi: 10.1124/jpet.106.111351. Epub 2006 Sep 19. J Pharmacol Exp Ther. 2006. PMID: 16985166
-
The infarct-sparing effect of IB-MECA against myocardial ischemia/reperfusion injury in mice is mediated by sequential activation of adenosine A3 and A 2A receptors.Basic Res Cardiol. 2015 Mar;110(2):16. doi: 10.1007/s00395-015-0473-x. Epub 2015 Feb 26. Basic Res Cardiol. 2015. PMID: 25711314
-
The A3 adenosine receptor agonist CP-532,903 [N6-(2,5-dichlorobenzyl)-3'-aminoadenosine-5'-N-methylcarboxamide] protects against myocardial ischemia/reperfusion injury via the sarcolemmal ATP-sensitive potassium channel.J Pharmacol Exp Ther. 2008 Jan;324(1):234-43. doi: 10.1124/jpet.107.127480. Epub 2007 Sep 28. J Pharmacol Exp Ther. 2008. PMID: 17906066 Free PMC article.
-
A3 adenosine receptor agonist IB-MECA reduces myocardial ischemia-reperfusion injury in dogs.Am J Physiol Heart Circ Physiol. 2003 Aug;285(2):H607-13. doi: 10.1152/ajpheart.01001.2002. Epub 2003 Apr 10. Am J Physiol Heart Circ Physiol. 2003. PMID: 12689858 Free PMC article.
-
IB-MECA and cardioprotection.Cardiovasc Drug Rev. 2006 Fall-Winter;24(3-4):227-38. doi: 10.1111/j.1527-3466.2006.00227.x. Cardiovasc Drug Rev. 2006. PMID: 17214599 Review.
Cited by
-
MicroRNA-21 Mediates Isoflurane-induced Cardioprotection against Ischemia-Reperfusion Injury via Akt/Nitric Oxide Synthase/Mitochondrial Permeability Transition Pore Pathway.Anesthesiology. 2015 Oct;123(4):786-798. doi: 10.1097/ALN.0000000000000807. Anesthesiology. 2015. PMID: 26259139 Free PMC article.
-
Adenosine A₂A and A₂B receptors are both required for adenosine A₁ receptor-mediated cardioprotection.Am J Physiol Heart Circ Physiol. 2011 Sep;301(3):H1183-9. doi: 10.1152/ajpheart.00264.2011. Epub 2011 Jul 8. Am J Physiol Heart Circ Physiol. 2011. PMID: 21743001 Free PMC article.
-
Regulation of leukocyte function by adenosine receptors.Adv Pharmacol. 2011;61:95-114. doi: 10.1016/B978-0-12-385526-8.00004-7. Adv Pharmacol. 2011. PMID: 21586357 Free PMC article. Review.
-
Chronic Co-Administration of Sepiapterin and L-Citrulline Ameliorates Diabetic Cardiomyopathy and Myocardial Ischemia/Reperfusion Injury in Obese Type 2 Diabetic Mice.Circ Heart Fail. 2016 Jan;9(1):e002424. doi: 10.1161/CIRCHEARTFAILURE.115.002424. Circ Heart Fail. 2016. PMID: 26763290 Free PMC article.
-
Adenosine A3 Receptor: From Molecular Signaling to Therapeutic Strategies for Heart Diseases.Int J Mol Sci. 2024 May 25;25(11):5763. doi: 10.3390/ijms25115763. Int J Mol Sci. 2024. PMID: 38891948 Free PMC article. Review.
References
-
- Auchampach JA, Rizvi A, Qiu Y, Tang XL, Maldonado C, Teschner S, et al. Selective activation of A3 adenosine receptors with N6-(3-iodobenzyl)adenosine-5′-N-methyluronamide protects against myocardial stunning and infarction without hemodynamic changes in conscious rabbits. Circ Res. 1997;80:800–9. - PubMed
-
- Ge ZD, Peart JN, Kreckler LM, Wan TC, Jacobson MA, Gross GJ, et al. Cl-IB-MECA [2-chloro-N6-(3-iodobenzyl)adenosine-5′-N-methylcarboxamide] reduces ischemia/reperfusion injury in mice by activating the A3 adenosine receptor. J Pharmacol Exp Ther. 2006;319:1200–10. - PubMed
-
- Jordan JE, Thourani VH, Auchampach JA, Robinson JA, Wang NP, Vinten-Johansen J. A3 adenosine receptor activation attenuates neutrophil function and neutrophil-mediated reperfusion injury. Am J Physiol. 1999;277:H1895–905. - PubMed
-
- Thourani VH, Nakamura M, Ronson RS, Jordan JE, Zhao ZQ, Levy JH, et al. Adenosine A3-receptor stimulation attenuates postischemic dysfunction through KATP channels. Am J Physiol. 1999;277:H228–35. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

