Sirtuin chemical mechanisms
- PMID: 20132909
- PMCID: PMC2886189
- DOI: 10.1016/j.bbapap.2010.01.021
Sirtuin chemical mechanisms
Abstract
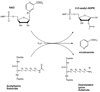
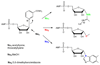
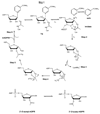
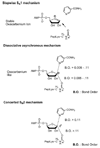
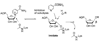
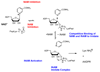
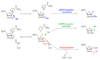
Sirtuins are ancient proteins widely distributed in all lifeforms of earth. These proteins are universally able to bind NAD(+), and activate it to effect ADP-ribosylation of cellular nucleophiles. The most commonly observed sirtuin reaction is the ADP-ribosylation of acetyllysine, which leads to NAD(+)-dependent deacetylation. Other types of ADP-ribosylation have also been observed, including protein ADP-ribosylation, NAD(+) solvolysis and ADP-ribosyltransfer to 5,6-dimethylbenzimidazole, a reaction involved in eubacterial cobalamin biosynthesis. This review broadly surveys the chemistries and chemical mechanisms of these enzymes.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures

References
-
- Sauve AA, Wolberger C, Schramm VL, Boeke JD. The biochemistry of sirtuins. Annu Rev Biochem. 2006;75:435–465. - PubMed
-
- Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–435. - PubMed
-
- Bishop NA, Guarente L. Genetic links between diet and lifespan: shared mechanisms from yeast to humans. Nat Rev Genet. 2007;8:835–844. - PubMed
-
- Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

