Iron uptake from plasma transferrin by a transferrin receptor 2 mutant mouse model of haemochromatosis
- PMID: 20133002
- PMCID: PMC2880807
- DOI: 10.1016/j.jhep.2009.12.010
Iron uptake from plasma transferrin by a transferrin receptor 2 mutant mouse model of haemochromatosis
Abstract
Background & aims: Hereditary haemochromatosis type 3 is caused by mutations in transferrin receptor (TFR) 2. TFR2 has been shown to mediate iron transport in vitro and regulate iron homeostasis. The aim of this study was to determine the role of Tfr2 in iron transport in vivo using a Tfr2 mutant mouse.
Methods: Tfr2 mutant and wild-type mice were injected intravenously with (59)Fe-transferrin and tissue (59)Fe uptake was measured. Tfr1, Tfr2 and ferroportin expression was measured by real-time PCR and Western blot. Cellular localisation of ferroportin was determined by immunohistochemistry.
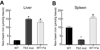
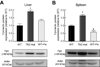
Results: Transferrin-bound iron uptake by the liver and spleen in Tfr2 mutant mice was reduced by 20% and 65%, respectively, whilst duodenal and renal uptake was unchanged compared with iron-loaded wild-type mice. In Tfr2 mutant mice, liver Tfr2 protein was absent, whilst ferroportin protein was increased in non-parenchymal cells and there was a low level of expression in hepatocytes. Tfr1 expression was unchanged compared with iron-loaded wild-type mice. Splenic Tfr2 protein expression was absent whilst Tfr1 and ferroportin protein expression was increased in Tfr2 mutant mice compared with iron-loaded wild-type mice.
Conclusions: A small reduction in hepatic transferrin-bound iron uptake in Tfr2 mutant mice suggests that Tfr2 plays a minor role in liver iron transport and its primary role is to regulate iron metabolism. Increased ferroportin expression due to decreased hepcidin mRNA levels is likely to be responsible for impaired splenic iron uptake in Tfr2 mutant mice.
Copyright (c) 2009 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Figures




References
-
- Kawabata H, Yang R, Hirama T, Vuong PT, Kawano S, Gombart AF, et al. Molecular cloning of transferrin receptor 2. A new member of the transferrin receptor-like family. J Biol Chem. 1999;274:20826–20832. - PubMed
-
- Graham RM, Reutens GM, Herbison CE, Delima RD, Chua AC, Olynyk JK, et al. Transferrin receptor 2 mediates uptake of transferrin-bound and non-transferrin-bound iron. J Hepatol. 2008;48:327–334. - PubMed
-
- Kawabata H, Germain RS, Vuong PT, Nakamaki T, Said JW, Koeffler HP. Transferrin receptor 2-alpha supports cell growth both in iron-chelated cultured cells and in vivo. J Biol Chem. 2000;275:16618–16625. - PubMed
-
- West AP, Jr, Bennett MJ, Sellers VM, Andrews NC, Enns CA, Bjorkman PJ. Comparison of the interactions of transferrin receptor and transferrin receptor 2 with transferrin and the hereditary hemochromatosis protein HFE. J Biol Chem. 2000;275:38135–38138. - PubMed
-
- Chen J, Chloupkova M, Gao J, Chapman-Arvedson TL, Enns CA. HFE modulates transferrin receptor 2 levels in hepatoma cells via interactions that differ from transferrin receptor 1-HFE interactions. J Biol Chem. 2007;282:36862–36870. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

